XIENCE™ Stent as the Gold Standard Among DES1
A wealth of clinical evidence supports the safety of XIENCE™ Stent. It’s a decisive reason why interventional cardiologists (ICs) continue to be confident that the acute outcomes they observe in their patients will persist over the long term. XIENCE Skypoint™ Stent—with design improvements and a 48-mm stent option to treat long lesions—represents the latest generation among the XIENCE™ Stents.
XIENCE™ Stent Safety Outcomes
XIENCE Skypoint™ Stent continues the legacy of outstanding safety outcomes of XIENCE™ Stent. It demonstrates consistency with optimal acute and early outcomes—in all types of patients and in complex lesions.2,3
| Types of Patients2,3 | Types of Lesions2,3 |
|---|---|
| Short DAPT | Bifurcations |
| Acute Myocardial Infarction (AMI) | Calcified Lesions |
| Diabetic | Long Lesions |

The 99% device success rate was the lowest success rate noted, even among a range of patient types in multiple clinical trials.2
Excellent Outcomes for Acute Myocardial Infarction (AMI)4
Early outcomes in AMI patients revealed that XIENCE™ Stent showed significant improvement 2 weeks after percutaneous coronary intervention (PCI). For example, during that timeframe the findings demonstrated:
- Better strut coverage (p < 0.01)
- Less thrombus formation (p = 0.03)
- Larger lumen area (p = 0.048)
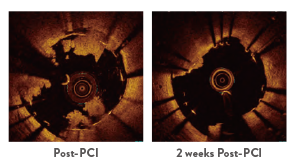
Representative OCT Images
XIENCE™ Stent also demonstrates safety among ST-elevation myocardial infarction (STEMI) patients, a subgroup of AMI patients. At 30 days, the rate of definite stent thrombosis (ST) with XIENCE™ Stent vs bare metal stents (BMS) was significantly lower: 0.4% vs 1.6%, p = 0.02.5
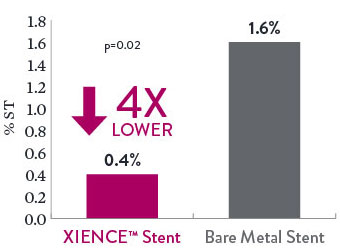
Definite ST in STEMI Patients
Excellent Outcomes in Complex Patients
In other types of complex patients, early (30-day) outcomes reveal very low rates of definite ST. This includes patients with bifurcations, calcified lesions, and long lesions.

Definite ST – 30 Days
Significantly Lower Acute, Early Stent Thrombosis
XIENCE™ Stent has significantly lower rates of acute and early ST than Synergy‡ and Resolute‡ DES.9,10
XIENCE™ Stent Acute ST Rate 75% Lower vs Other DES
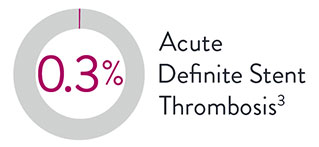
There is a 75% lower rate of acute (24-hour) definite ST with XIENCE™ Stent vs Synergy‡.9
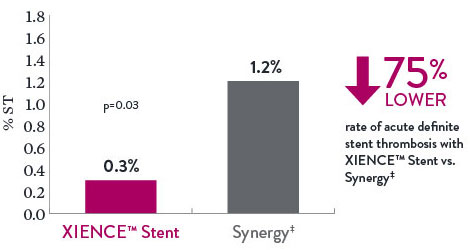
Acute Definite Stent Thrombosis
XIENCE™ Stent ST Rate 88% Lower vs Other DES
There is an 88% lower rate of early (30-day) definite ST with XIENCE™ Stent vs Resolute‡.10
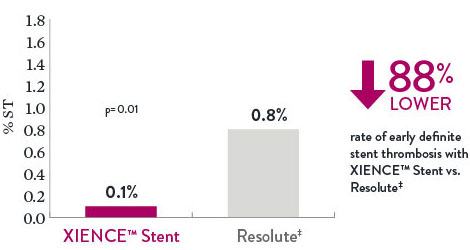
Definite Stent Thrombosis 30 Day
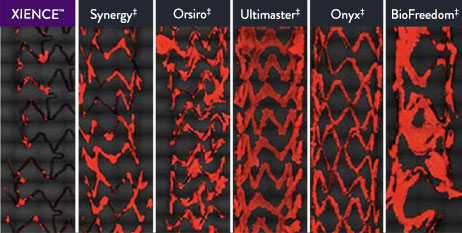
XIENCE™ Stent Is More Anti-Thrombotic Than Other DES11
Platelet adhesion is a key factor in stent thrombosis, and the XIENCE™ Stent’s unique, durable fluoropolymer was created to ensure long-term protection from thrombosis.
Preclinical testing confirms that the XIENCE™ Stent exhibits the least platelet adhesion compared to other DES (p < 0.01). This factor protects patients from short-term complications.11
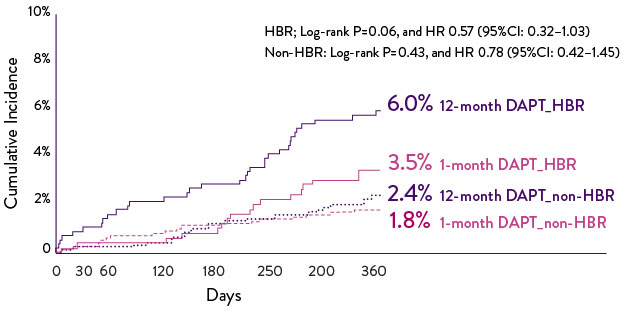
High Bleeding Risk Patients: Low Event Rates at 1 Year12
One-year subgroup analysis data from the STOPDAPT-2 trial showed excellent patient outcomes for high bleeding risk (HBR) patients using 1-month dual antiplatelet therapy (DAPT). Patients at high bleeding risk who followed a 1-month DAPT regimen had significantly lower event rates than those using a 12-month DAPT strategy.
Evidence of Unparalleled Outcomes Long After PCI1
Extensive evidence demonstrates that XIENCE™ Stents deliver unparalleled patient outcomes which last far beyond the intervention. Data from multiple studies support these exceptional results.

References
- Zanchin C, et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675. Serruys P, et al. N Engl J Med. 2010;363:136-146. Shiomi H, et al. JACC Cardiovasc Interv. 2019;12:637-647. Kufner S, et al. Circulation. 2019:139(3):325-333. Palmerini T, et al. Lancet. 2013;379:1393-1402. Bangalore S, et al. Circulation. 2012;125:2873-2891. Bangalore S, et al. Circ Cardiovasc Interv. 2013;6(6):378-390. Pilgrim T, et al. Lancet. 2014;384:2111-2122. Pilgrim T, et al. Lancet. 2018;392:737-746. Data on file at Abbott.
- Saito S, et al. EuroIntervention. 2019;15(11):e1006-e1013. Saito S, et al. Eur Heart J. 2014;35:2021-2031. Natsuaki M, et al. J Am Coll Cardiol. 2013;62:181-190. Stone G, et al. Lancet. 2018;392:1530-1540. *Note: 99% was the lowest device success rate.
- Zanchin C, et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675. Serruys P, et al. N Engl J Med. 2010;363:136-146. Watanabe H, et al. JAMA. 2019;321(24):2414-2427.
- Morino Y, et al. Cardiovasc Interv Ther. 2019;34(1):14-24.
- Sabate M, et al. Lancet. 2012;380:1482-1490.
- Džavík V, et al. Catheter Cardiovasc Interv. 2013;82(3):E163-E172.
- Onuma Y, et al. Catheter Cardiovasc Interv. 2010;76:634-642.
- Hong S, et al. JACC Cardiovasc Interv. 2016;9:1438-1446.
- Zanchin C, et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675.
- Serruys P, et al. N Engl J Med. 2010;363:136-146.
- Jinnouchi H, et al. J Am Coll Cardiol. 2019;74:(Suppl B):TCT-291. Zanchin, C. et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675.
- Watanabe H, et al. Cardiovasc Interv and Ther. 2021;36:91-103.
- Von Birgelen C, et al. J Am Coll Cardiol. 2012;59(15):1350-1361.
- Van Geuns RJ, et al. TCT 2019, IDEAL-LM.
- Chevalier B, et al. EuroIntervention. 2018;13(13):1561-1564.
- Gao R, et al. TCT 2019, ABSORB China.
MAT-2107574 v3.0
