Expanded Treatment Options2
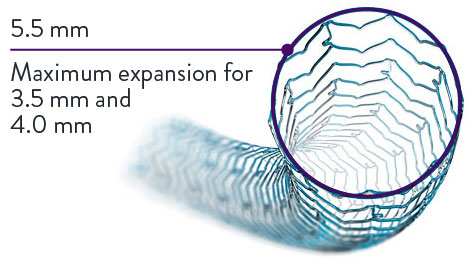
Uniquely Designed to Post-Dilate up to 5.5 mm
With 5.5 mm post-dilatation capability for 3.5 mm and 4.0 mm stents, XIENCE Sierra™ Stent can treat large vessels.
And with 3.75-mm post-dilatation capability for smaller diameter stents—2.0 mm, 2.25 mm, 2.5 mm, 2.75 mm, 3.0 mm, and 3.25 mm—XIENCE Sierra™ Stent can also treat tapered vessels of various diameters.
These options for stent diameters and post-expansion diameters provide a range of choices to treat the array of vessels that interventional cardiologists (ICs) encounter.
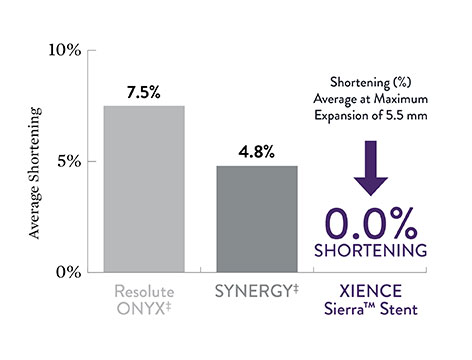
No Shortening for Unsurpassed Precision in Placement
XIENCE Sierra™ Stent exhibits no shortening even at max expansion of 5.5 mm.3 This assists ICs by:
- Providing precision in placement
- Avoiding a geographic miss
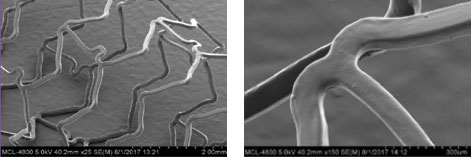
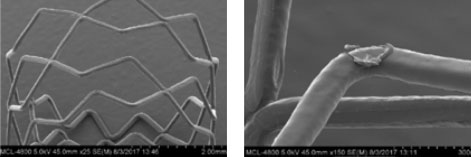
XIENCE™ Stent Design Ensures Coating Integrity4
For images below, the left visual is magnified 25x and the right visual is magnified 150x.
XIENCE™ Stent Coating
XIENCE™ Stent coating remains intact at maximum post-dilatation expansion of 5.5 mm from 3.5 mm (3.5 x 18 mm).
Synergy‡ Coating
The Synergy‡ coating shows multiple cracks with delamination at its max expansion of 4.25 mm from 3.5 mm (3.5 x 20 mm).
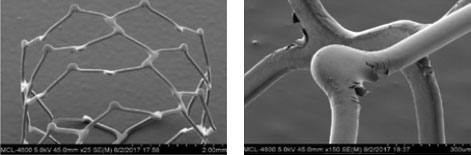
Resolute Onyx‡ Coating
The Resolute Onyx‡ coating peels off and shows exposed metal at its max expansion of 4.75 mm from 3.5 mm (3.5 x 18 mm).
References
- Zanchin C, et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675. Serruys P, et al. N Engl J Med. 2010;363:136-146. Shiomi H, et al. JACC Cardiovasc Interv. 2019;12:637-647. Kufner S, et al. Circulation. 2019:139(3):325-333. Palmerini T, et al. Lancet. 2013;379:1393-1402. Bangalore S, et al. Circulation. 2012;125:2873-2891. Bangalore S, et al. Circ Cardiovasc Interv. 2013;6(6):378-390. Pilgrim T, et al. Lancet. 2014;384:2111-2122. Pilgrim T, et al. Lancet. 2018;392:737-746. Data on file at Abbott.
- XIENCE Sierra™ Stent - Instructions for Use (IFU). Refer to IFU for additional information. Increased maximum expansion compared to other XIENCE™ Everolimus Eluting Coronary Stent System.
- Data on file at Abbott. XIENCE Sierra™ (4.0 x 18 mm, n=5), SYNERGY‡ (4.0 x 20 mm, n=5), Resolute Onyx‡ (4.5 x 18 mm, n=5). All stents deployed to 5.5mm.
- Data on file at Abbott
MAT-2101791 v2.0
