Excellent Deliverability with XIENCE Skypoint™ Stent2
The latest design elements, plus proven ability to deliver with previous generations of XIENCE™ Stents, provide excellent deliverability for interventional cardiologists (ICs) encountering challenging lesions.

Catheter Enhancements
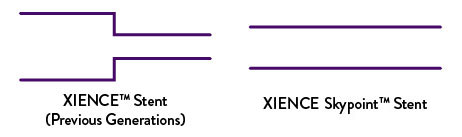
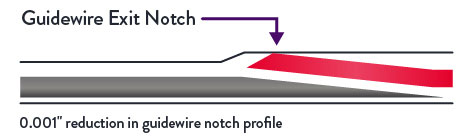
Unlike prior generations of XIENCE™ Stent, XIENCE Skypoint™ Stent has a seamless one-piece catheter shaft that optimizes pushability.2 In addition, the guide wire notch profile is reduced by 0.001”.3
Excellent Deliverability
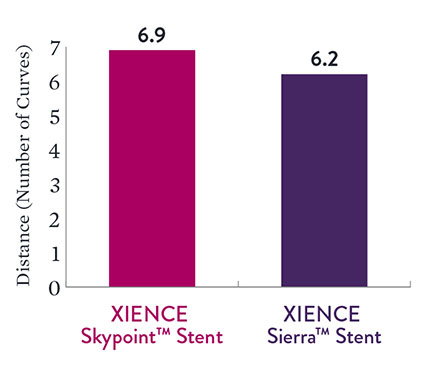
Data reveal excellent deliverability with XIENCE Skypoint™ Stent. This compares favorably with XIENCE Sierra™ Stent.4
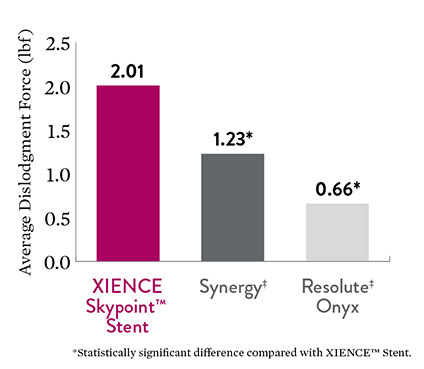
Better Stent Retention
High stent retention helps prevent dislodgement and help ensure safe crossing, even when treating complex lesions. Testing reveals that XIENCE Skypoint™ Stent force is better than those of Synergy‡ and Resolute Onyx‡ DES, reaching statistical significance.5
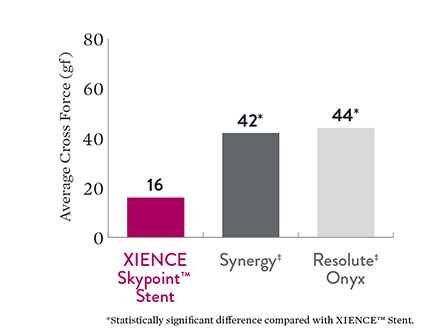
Lower Crossing Forces with XIENCE Skypoint™ Stent6
Lower crossing forces can also aid in optimizing stent deliverability. Note that XIENCE Skypoint™ Stent crossing forces are significantly lower than those of Synergy‡ and Resolute Onyx‡ DES.
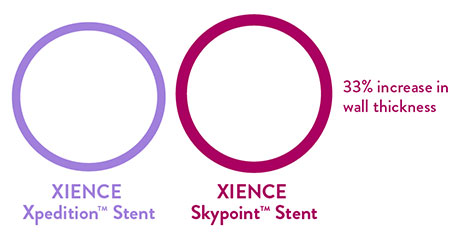
When using the XIENCE Skypoint™ 48-mm Stent, the hypotube has a 33% increase in wall thickness.7
Excellent Push Efficiency with XIENCE Skypoint™ Stent8
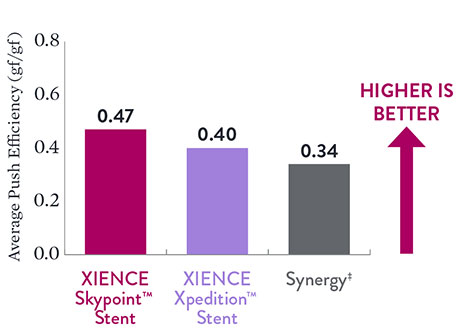
Push Efficiency–48 mm stents
References
- Zanchin C, et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675. Serruys P, et al. N Engl J Med. 2010;363:136-146. Shiomi H, et al. JACC Cardiovasc Interv. 2019;12:637-647. Kufner S, et al. Circulation. 2019:139(3):325-333. Palmerini T, et al. Lancet. 2013;379:1393-1402. Bangalore S, et al. Circulation. 2012;125:2873-2891. Bangalore S, et al. Circ Cardiovasc Interv. 2013;6(6):378-390. Pilgrim T, et al. Lancet. 2014;384:2111-2122. Pilgrim T, et al. Lancet. 2018;392:737-746. Data on file at Abbott.
- Data on file at Abbott—XIENCE Skypoint™ Stent compared with XIENCE Sierra™ Stent.
- Data on file at Abbott—XIENCE Skypoint™ Stent Product Specification.
- Test performed by and data on file at Abbott—3.0 x 18 mm or 3.0 x 20 mm stents tested using a simulated arterial model.
- Tests performed by and data on file at Abbott. XIENCE Skypoint™ Stent (3.0 x 18 mm) n=5, SYNERGY‡ (3.0 x 20 mm) n=5, Resolute Onyx‡ (3.0 x 18 mm) n=5.
- Data on file at Abbott. XIENCE Skypoint™ Stent (3.0 x 18 mm) n=5, SYNERGY‡ (3.0 x 20 mm) n=5, Resolute Onyx‡ (3.0 x 18 mm) n=5.
- Data on file at Abbott. Hypotube wall thickness information—XIENCE Skypoint™ Stent 0.004ʺ compared with XIENCE Xpedition™ Stent 0.003ʺ.
- Data on file at Abbott. XIENCE Skypoint™ Stent compared with XIENCE Xpedition™ Stent – 3.0 x 48 mm stents tested.
MAT-2107573 v2.0
