XIENCE Skypoint™ Stent: Better Expansion, Excellent Deliverability, and Trusted Patient Outcomes2-4
As the latest generation in the XIENCE™ family of stents, the stent platform that consistently delivers successful patient outcomes – not only in the cath lab, but also long-term.4 This stent also offers:
- The broadest expansion range among XIENCE™ Stents2
- The largest size range for a single XIENCE™ Stent product, from
2 mm to 5 mm in diameter and from 8 mm to 48 mm in length2
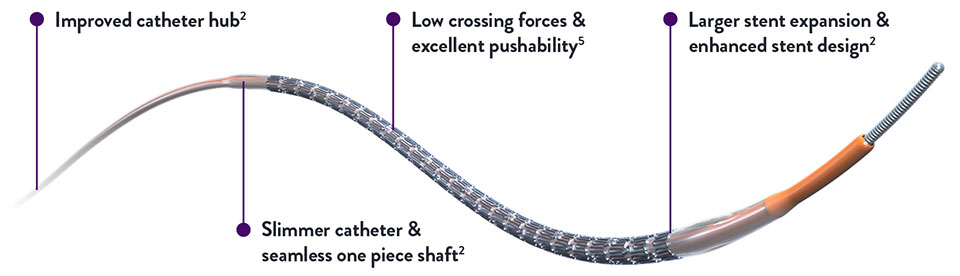
Stent Choice Matters
During percutaneous coronary interventions (PCI), small differences between stents can be the determining factor for the patient’s acute and long-term outcomes.4 XIENCE Skypoint™ Stent has a number of features that offer broader treatment options to interventional cardiologists (ICs).
Updated features include:
- Larger stent expansion and enhanced stent design2
- Slimmer catheter profile with integrated one-piece shaft2
- Low crossing forces and excellent pushability5
- Improved catheter hub2
- Improved stent design for 48-mm sizes2
- Now available in 4.5 and 5.0 mm diameter stent sizes2
These new features can help clinicians treat the increasingly wide range of lesions encountered in real-world patients.
XIENCE Skypoint™ Stent Retains Proven Design Elements
Compared to other DES, several key factors account for XIENCE™ Stent’s unparalleled clinical outcomes.1
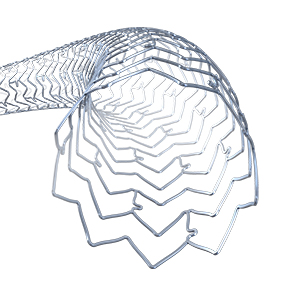
Innovative Stent Design:
The XIENCE™ Stent is designed to conform to vessel anatomy,6 as well as to reduce inflammation and minimize thrombogenicity7 Get more details about the stent design.
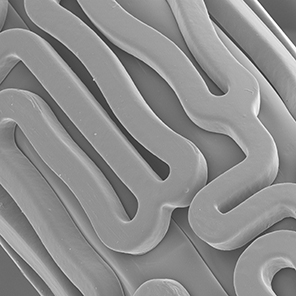
Thromboresistant Fluoropolymer:
Unlike other stents’ polymer coatings, the Abbott fluoropolymer interacts with blood proteins in a manner that reduces thrombus formation.7 View the visual evidence of fluoropolymer safety—significantly less inflammation and thrombus formation—compared to competitors’ stents.
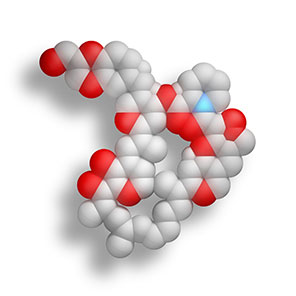
Market-Leading Everolimus:
The choice for XIENCE™ Stent is the drug everolimus. This broadly-used therapeutic drug is safe and stable over the long term2 See how the everolimus elution correlates to the restenosis cascade.
References
- Zanchin C, et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675. Serruys P, et al. N Engl J Med. 2010;363:136-146. Shiomi H, et al. JACC Cardiovasc Interv. 2019;12:637-647. Kufner S, et al. Circulation. 2019:139(3):325-333. Palmerini T, et al. Lancet. 2013;379:1393-1402. Bangalore S, et al. Circulation. 2012;125:2873-2891. Bangalore S, et al. Circ Cardiovasc Interv. 2013;6(6):378-390. Pilgrim T, et al. Lancet. 2014;384:2111-2122. Pilgrim T, et al. Lancet. 2018;392:737-746. Data on file at Abbott.
- Data on file Abbott.
- Data on file at Abbott—3.0 x 18 mm or 20 mm stents tested using a simulated arterial model. Comparison is between XIENCE Skypoint™ Stent, XIENCE Sierra™ Stent, Synergyǂ and Resolute Onyxǂ. Push efficiency comparison is between XIENCE Skypoint™ 48-mm Stent, XIENCE Xpedition™ 48-mm Stent and Synergyǂ 48 mm.
- Zanchin C, et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675. Serruys P, et al. N Engl J Med. 2010;363:136-146. Shiomi H, et al. JACC Cardiovasc Interv. 2019;12:637-647. Kufner S, et al. Circulation. 2019:139(3):325-333.
- Data on file at Abbott. Crossing force comparison is between XIENCE Skypoint™ Stent, Synergyǂ and Resolute Onyxǂ—3.0 x 18 mm or 20 mm stents tested. Push efficiency comparison is between XIENCE Skypoint™ Stent, XIENCE Xpedition™ Stent, and Synergyǂ – 3.0 x 48 mm for all stents tested.
- Colombo A, et al. J Am Coll Cardiol. 2002;40:1021-1033.
- Jinnouchi H, et al. J Am Coll Cardiol. 2019;74:(Suppl B):TCT-291.
MAT-2107569 v4.0

