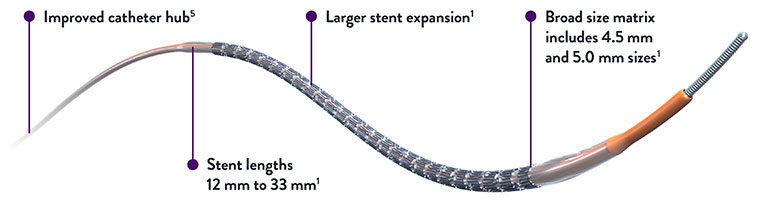
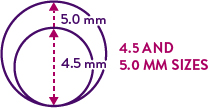
XIENCE Skypoint™ Stent Broad Size Matrix Includes 4.5 mm and 5.0 mm Sizes for the Treatment of Large Vessels
XIENCE Skypoint™ Stent the proven DES in a wide range of sizes that consistently delivers successful outcomes not only in the cath-lab, but far beyond.1,2
Treat a Wide Range of Large Vessel Lesions With XIENCE Skypoint™ Stents1
XIENCE Skypoint™ Stent offers a range of stent lengths from 12 mm to 33 mm to help treat a wide range of patients.1
12 mm
to
33 mm

| Stent Diameter | Stent Length | ||||||
| 12 mm | 15 mm | 18 mm | 23 mm | 28 mm | 33 mm | Maximum Expansion | |
| 4.5 mm | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 5.75 mm |
| 5.0 mm | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 5.75 mm |
XIENCE Skypoint™ Stent Shows Accurate Stent Placement With Precise Stent and Marker Placement4

Accurate mid-marker placement enables precise scaffolding and reduces the likelihood of geographic miss to support optimal patient outcomes.4,5
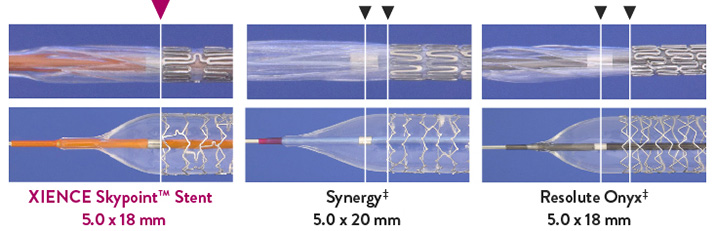
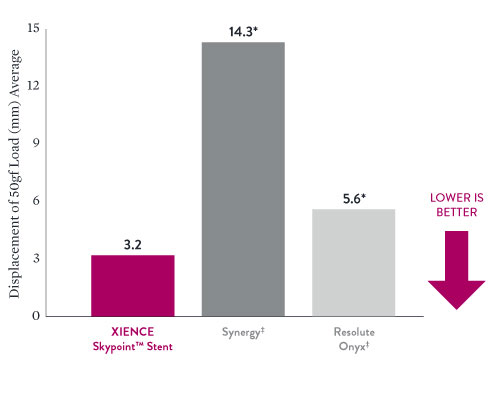
XIENCE Skypoint™ Stent has Significantly Better Longitudinal Strength Than Other DES in Large Vessels6
Longitudinal strength supports better scaffolding in large coronary vessels. Less stent deformation delivers better patient outcomes.7
XIENCE Skypoint™ Stent Shows Significantly Less Stent Deformation Than
Synergy‡ and Resolute Onyx‡ – 5.0 mm Stents
XIENCE™ Stent Shows Improvements in Measures of Quality of Life (QoL) in Patients With Left Main Lesions at 3 Years8
PCI With XIENCE™ Stent in patients with left main lesions showed sustained improvements in QoL out to 3 years8
SAQ - Quality of Life*
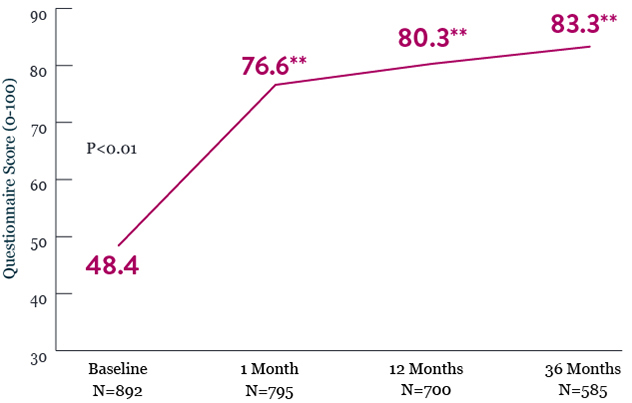
*Quality of life is a subscale of the Seattle Angina Questionnaire (SAQ). Scores for each subscale range from 0 to 100 with higher scores representing better health status. Comparison is made Vs baseline.
** Denotes statistically significant difference from baseline.

Frequency of Angina-Free Incidences as Measured by SAQ - AF*
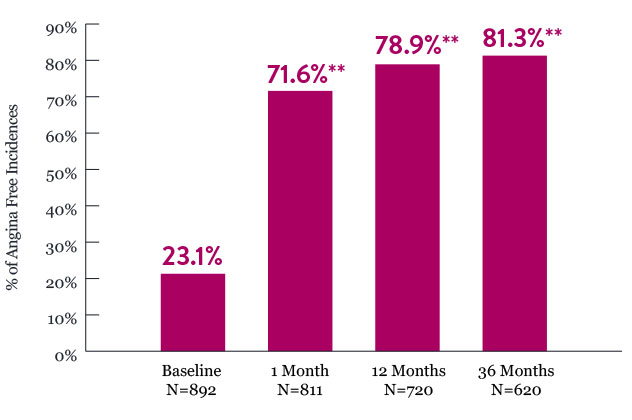
* Frequency of Angina is a subscale of the Seattle Angina Questionnaire (SAQ). Comparison is made vs baseline.
** Denotes statistically significant difference from baseline.
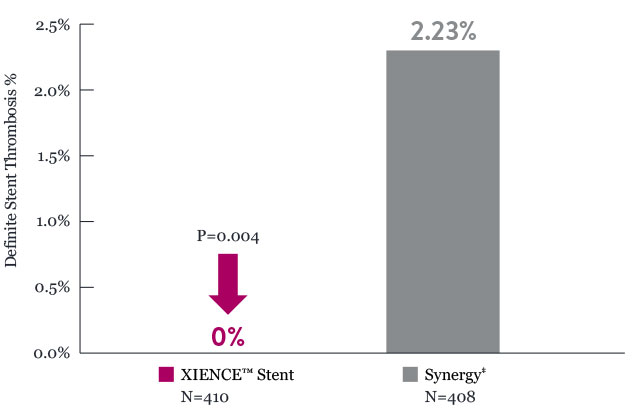
XIENCE™ Stent shows significantly lower definite stent thrombosis rates than Synergy‡ in patients with left main lesions at 2 years9
References
- XIENCE Skypoint™ Stent Instructions For Use (IFU). Refer to IFU for additional information.
- Zanchin, C., et al. J Am Coll Cardiol Intv. 2019;12(17):1665-1675. Serruys P, et al. N Engl J Med. 2010;363:136-146. Kufner S, et al. Circulation. 2019:139(3):325-333.
- Data on file at Abbott. XIENCE Skypoint™ Stent vs. Synergy‡ and Resolute Onyx‡ – 5.0 x 18 or 20 mm stents tested.
- Data on file at Abbott.
- Costa, M., et al. Am J Cardiol. 2008;101:1704-1711; Sudhir, K., et al. ISRN Cardiology. 2013:1-8.
- Data on file at Abbott. XIENCE Skypoint™ Stent – 5.0 x 33 mm, n=5. Synergy‡ 5.0 x 32 mm, n=2. Resolute Onyx‡ – 5.0 x 33 mm , n=5. *Denotes significantly different when compared with XIENCE Skypoint™ Stent. Longitudinal stent compression test was done using 50 gf load.
- Shand, J., et al. Interv Cardiol. 2012;4:325-335.
- Baron, S., et al. J Am Coll Cardiol. 2017;70:3113-3122.
- Van Geuns, R., et al. IDEAL-LM. TCT 2019.
MAT-2208522 v2.0
