Optimal Outcomes in Complex Patients
Reproducible outcomes are a proven part of XIENCE™ DES performance, delivering consistent patient safety with low complication rates. As a result, XIENCE™ Stent can treat lesions of any complexity in patients with a range of challenging comorbidities1
Increasingly Complex Patients Now Treated with PCI
Complex lesions and increasingly complex percutaneous coronary intervention (PCI) cases compose an ever-growing segment of the patient population, and stenting for these populations may expand in the future.
Even when patients are complex, choosing a stent shouldn't be. XIENCE Skypoint™ Stent is an excellent choice for complex patients2-5 given its best-in-class deliverability6 and expanded treatment options7.
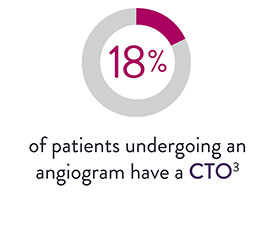
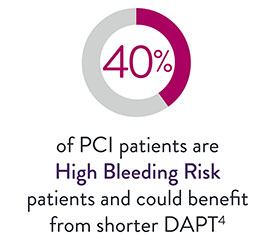
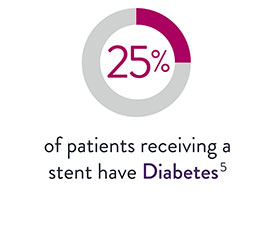
XIENCE™ Stent for CTO Lesions
XIENCE™ Stent, the world’s leading DES, is considered by industry experts to be the gold standard.8 It offers unmatched consistency and optimal acute outcomes in all types of patients and lesions, including complex cases.9,10


XIENCE™ Stent Complex Patient Data: Consistently Low Stent Thrombosis Rates11-16
Results from a number of multicenter, randomized trials show that XIENCE™ Stent has low stent thrombosis (ST) rates, from 30 days to 5 years.
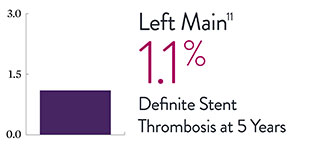
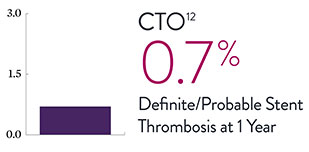
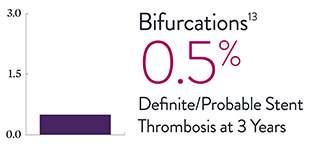
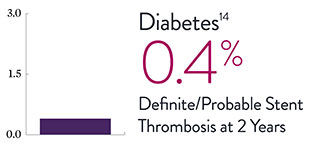
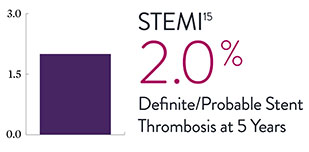
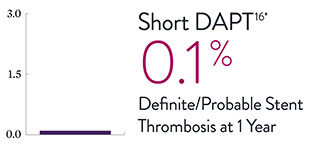
* DAPT duration of 1 month
XIENCE™ Stent in All-Comer Complex Patient Population
In a meta-analysis of all-comer complex patients, XIENCE™ Stent was shown to save lives compared to bare metal stents (BMS).17
2-Year Clinical Outcomes: XIENCE™ Stent vs Bare Metal Stents17
Fatal Myocardial Infarction
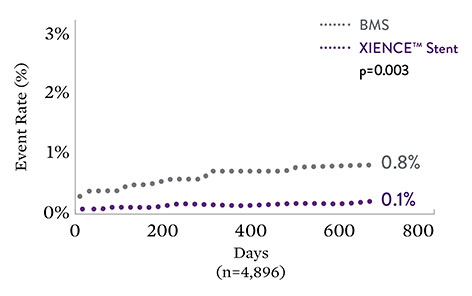
XIENCE™ Stent results in an 89% risk reduction
Primary Endpoint: Cardiac Death
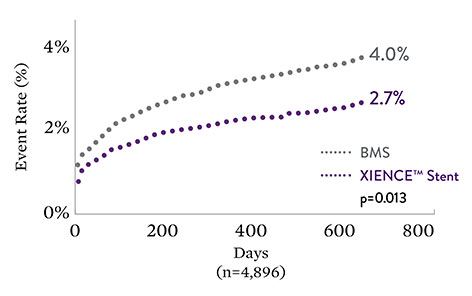
XIENCE™ Stent results in a 33% risk reduction vs BMS
| Myocardial Infarction | BMS (%) | DES (%) | p value (non-adjusted) | p value (†adjusted) |
|---|---|---|---|---|
| All | 5.6% | 4.0% | 0.01 | 0.01 |
| Non-fatal | 4.8% | 3.9% | 0.12 | 0.12 |
| Fatal | 0.8% | 0.1% | 0.003 | 0.004 |
†Adjusted for clinical syndrome (ie, acute coronary syndrome vs stable syndrome), history of diabetes mellitus, female sex, use of glycoprotein IIb/IIIa inhibitors, and up to 1 year vs longer duration of DAPT.
“The benefit of [XIENCE™ Stents] occurred immediately after implantation and . . . also at long-term follow-up beyond 2 years.”
—Manel Sabaté, MD, on the EXAMINATION trial's lower event rates for XIENCE™ Stent versus bare metal stents in STEMI patients15
Detailed Data on Complex Patients, Complex Lesions
XIENCE™ Stent is increasingly used—and evaluated via clinical trials—in complex patients and when treating complex lesions. View more data on complex patients: short DAPT and AMI.
References
- Zanchin C, et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675. Serruys P, et al. N Engl J Med. 2010;363:136-146. Shiomi H, et al. JACC Cardiovasc Interv. 2019;12:637-647. Kufner S, et al. Circulation. 2019:139(3):325-333. Palmerini T, et al. Lancet. 2013;379:1393-1402. Bangalore S, et al. Circulation. 2012;125:2873-2891. Bangalore S, et al. Circ Cardiovasc Interv. 2013;6(6):378-390. Pilgrim T, et al. Lancet. 2014;384:2111-2122. Pilgrim T, et al. Lancet. 2018;392:737-746. Data on file at Abbott.
- Shaw JE, et al. Diabetes Res Clin Pract. 2010;87:4-14.
- Fefer P, et al. J Am Coll Cardiol. 2012;59(11):991-997.
- Capodanno D, et al. J Am Coll Cardiol. 2020;76:1468–1483.
- Armstrong E, et al. J Diabetes Sci Technol. 2014;8(3):581–589.
- Data on file at Abbott. Bench test data shows XIENCE Skypoint™ Stent (3.0 x 18 mm, n=5) performance compared to Resolute Onyx‡ (3.0 x 18mm, n=5) , SYNERGY MONORAIL‡ (3.0 x 20 mm, n=5) , Promus Elite‡ (3.0 x 20 mm, n=5) , BIOFREEDOM‡ (3.0 x 18 mm, n=5), Resolute Onyx‡ (3.0 x 18 mm, n=5) and ORSIRO‡ (3.0 x 18 mm, n=5). Bench test results may not necessarily be indicative of clinical performance. Catheter test measures average force to cross a challenging lesion model. XIENCE Skypoint™ Stent performed better in crossability and was not statistically different in trackability and pushability.
- Instructions for Use – XIENCE Skypoint™ Stent – increased maximum expansion compared to XIENCE Sierra™ Stent.
- Data on file at Abbott – XIENCE™ Stent data includes 120 clinical trials, 10+ years of data, 125,000 patients studied, 20,000,000 implants as of Q4 2022 and is the most implanted DES worldwide.
- Saito S, et al. EuroIntervention. 2019;15(11):e1006-e1013. Saito S, et al. Eur Heart J. 2014;35:2021-2031. Natsuaki M, et al. J Am Coll Cardiol. 2013;62:181-190. Stone G, et al. TCT 2017 – ABSORB IV. Note: 99% is the lowest device success rate.
- Serruys P, et al. N Engl J Med. 2010;363:136-146. Zanchin C. et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675. Note: 0.3% is the highest definite stent thrombosis rate.
- Stone G, et al. N Engl J Med. 2019;381:1820-1830.
- Teeuwen K, et al. JACC Cardiovasc Interv. 2017;10:133-143.
- Lam MK, et al. Am Heart J. 2015;169:69-77.
- Kaul U, et al. EuroIntervention. 2017;13:1194-1201.
- Sabaté M, et al. Lancet. 2016;387;357-366.
- Watanabe H, et al. JAMA. 2019;321(24):2414-2427.
- Valgimigli M, et al. BMJ. 2014;349:g6427.
MAT-2101789 v2.0
