XIENCE™ Stent: Protecting Patients with Short DAPT Needs
Dual antiplatelet therapy (DAPT) duration often depends on the patient—and it may depend on the stent as well.
XIENCE™ Stent Has the Largest Body of DAPT Patient Evidence3
XIENCE™ Stent is the only DES that is supported by 0, 1 and 3 months of DAPT data. XIENCE™ Stent is the only DES with results showing 1-month DAPT data and 3-month DAPT data for both Aspirin and P2Y12 inhibitor monotherapy—including data on patients at high bleeding risk (HBR).4
Among all 3 categories outlined above—aspirin monotherapy, P2Y12 inhibitor monotherapy and HBR patients—XIENCE™ Stent data revealed very low rates of stent thrombosis (ST) at 12 months.5
≤ 0.3% Definite ST At 1 Year5
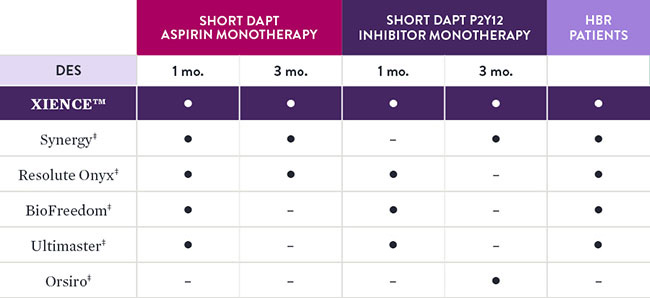
Comparison of Short DAPT Studies by Stent Type4
XIENCE 28 and XIENCE 90 Study Results6
XIENCE™ Stent Short DAPT: Ischemic Events
Among HBR patients, XIENCE™ Stent with 1-month or 3-month DAPT reduced severe bleeding with no increase in ischemic events, including myocardial infarction (MI) and all death.6
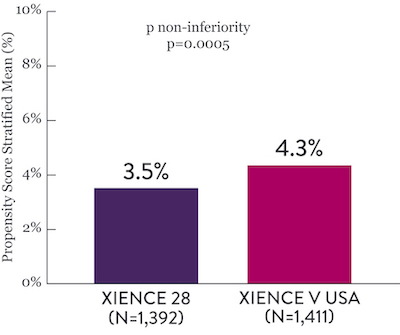
XIENCE 28: 1-month DAPT in HBR Patients
XIENCE 28: All Death or MI
Between 1 and 6 months
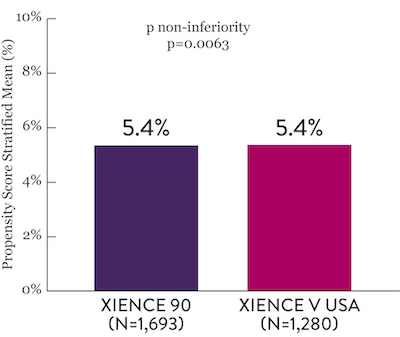
XIENCE 90: 3-month DAPT in HBR Patients
XIENCE 90: All Death or MI
Between 3 and 12 months
XIENCE™ Stent Short DAPT: Reduced Severe Bleeding
In the same HBR population, XIENCE™ Stent with 1-month or 3-month DAPT reduced severe bleeding with no increase in ischemic events.6,*
XIENCE 28: BARC 3-5 Bleeding
Between 1 and 6 months
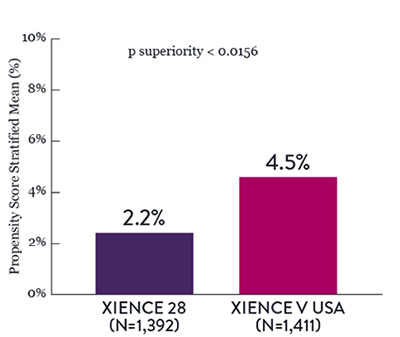
XIENCE 90: BARC 3-5 Bleeding
Between 3 and 12 months
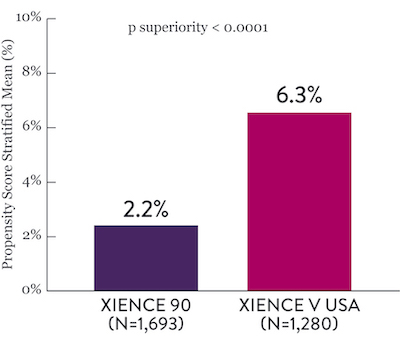
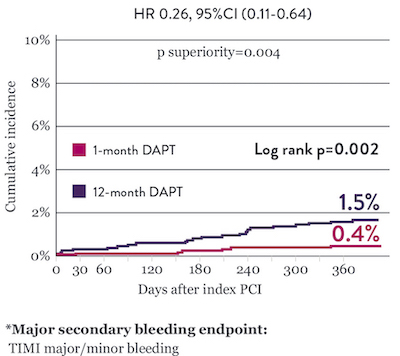
*Propensity score stratified analysis for BARC 3-5 bleeding was not pre-specified. BARC 2-5 was a powered secondary endpoint. In both studies, for BARC 2-5, XIENCE™ Stent showed numerically lower bleeding rate for 1-month or 3-month DAPT versus 6-month DAPT or 12-month DAPT, respectively.
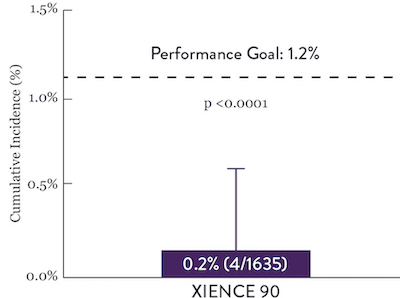
XIENCE™ Stent Short DAPT: Continued Low Stent Thrombosis
XIENCE™ Stent is recognized for its low ST rate, and it is significantly more anti-thrombotic than other DES.7 This is evident even with short DAPT data. XIENCE™ Stent with 1-month DAPT showed no increase in ST vs 6-month DAPT—with an ST rate of 0.3%. Similarly, the 3-month DAPT showed an ST rate of 0.2%.6
XIENCE 28: Stent Thrombosis
Between 1 and 6 months
ARC : Definite/Probable ST
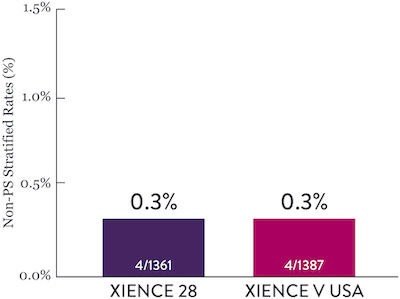
XIENCE 90: Stent Thrombosis
Between 3 and 12 months
ARC : Definite/Probable ST
XIENCE™ Stent Is Anti-Thrombotic: Suited for Short DAPT
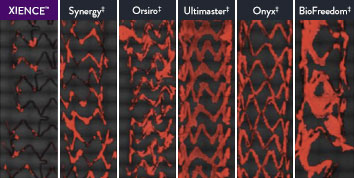
The XIENCE™ Stent is also recognized as being significantly more anti-thrombotic than other DES on the market. As shown in the study findings, XIENCE™ Stent reveals significantly less (p < 0.01) platelet adhesion—shown in red in the confocal microscopy images—than other DES, and platelet adhesion is an important factor in stent thrombosis.*8 These findings suggest that this stent choice “may be ideally suited for very short-term DAPT.”8
*Ex Vivo Swine Shunt Model.
STOPDAPT Studies: 1-Month DAPT and 3-Month DAPT in All-Comers Population9,10
STOPDAPT9 and STOPDAPT 210 were prospective trials of the XIENCE™ Stent that studied DAPT cessation at 3 months and 1 month, respectively
STOPDAPT 2 Trial: 1-Month DAPT Superior to 12-Month DAPT10
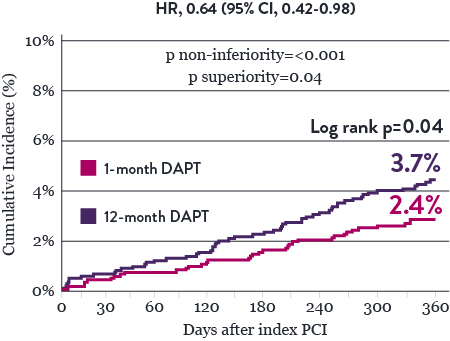
The STOPDAPT 2 trial revealed that 1-month DAPT demonstrated superior safety over 12-month DAPT for the primary endpoint of net adverse cardiovascular events (NACE). NACE included cardiovascular death, myocardial infarction (MI), definite ST, stroke, or thrombolysis in MI (TIMI) major/minor bleeding. All of the 3,009 patients in this randomized, controlled trial were treated with XIENCE™ Stent.10
Significantly Lower NACE* with 1-Month DAPT
Significantly Lower Bleeding Events* with 1‑Month DAPT
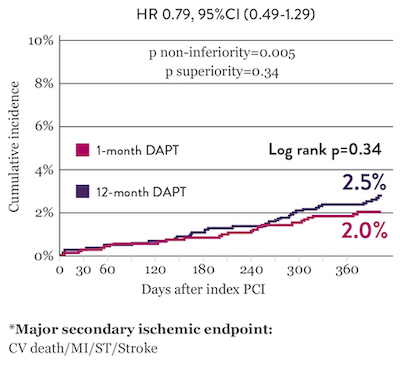
Comparable Ischemic Event Rates* with 1‑Month DAPT
“Stopping DAPT at 3 months in selected patients after [XIENCE™ Stent] implantation was at least as safe as the prolonged DAPT regimen adopted in the historical control group.”
— Masahiro Natsuaki, MD, STOPDAPT Trial9


STOPDAPT 2 Trial Design and Randomization10
Short 1-Month DAPT
- 0 to 1-month: Aspirin + P2Y12
- After 1-month: Clopidogrel monotherapy

12-Month DAPT
- 0 to 1-month: Aspirin + P2Y12
- 1 to 12-month: Aspirin + Clopidogrel
- 12 to 60-month: Aspirin monotherapy

- Successful PCI using CoCr everolimus-eluting stent: XIENCE™
- Eligible for DAPT (aspirin/P2Y12 receptor blocker) for 1 year

- Patients who need oral anticoagulants
- History of intracranial bleeding
- Major in-hospital complications (MI/stroke/major bleeding)

STOPDAPT Study: XIENCE™ Stent with 3-Month DAPT Is Feasible9
STOPDAPT9 was the first prospective trial to study DAPT cessation at 3 months after implantation. Among other 1-year outcomes, the XIENCE™ Stent rate of stent thrombosis was 0.0%.
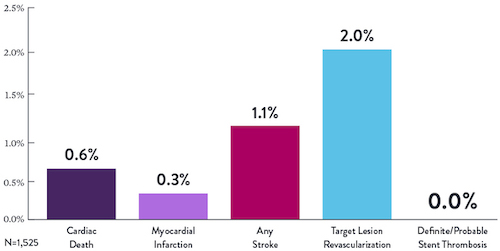
“It was noteworthy that no definite or probable stent thrombosis occurred in [XIENCE™ Stent] patients enrolled in STOPDAPT.”
— Masahiro Natsuaki, MD, STOPDAPT Trial9
STOPDAPT-3 Trial Design and Randomization11
- PCI with planned exclusive use of CoCr-EES (XIENCE)
- ACS presentation or ARC-HBR
- Eligible for DAPT (Aspirin/P2Y12inhibitor) for 1 month

Study design and Randomization
Group 1:
0 to 1-month: Aspirin + P2Y12 (Prasugrel)
After 1-month: Clopidogrel monotherapy
Group 2:
0 to 1-month: P2Y12 (Prasugrel)
After 1-month: Clopidogrel monotherapy
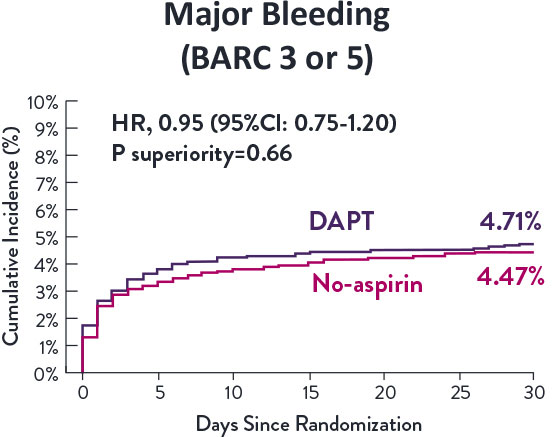
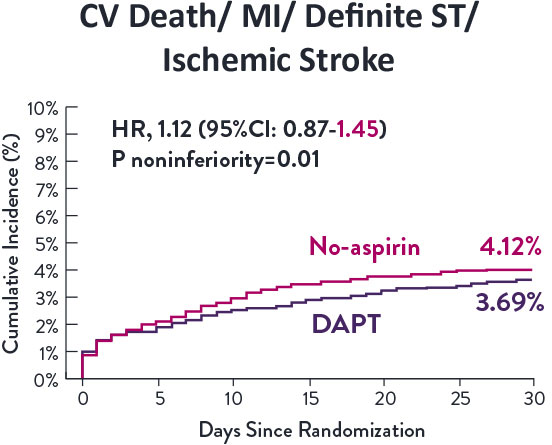
STOPDAPT-3 Trial 11 was designed to explore 0-month DAPT* (SAPT˄ using only P2Y12 inhibitor) for ACS and HBR patients.
Though the results are comparable for both bleeding and ischemic events for DAPT and SAPT arms, the study did not meet its endpoint and concluded to use DAPT for 1 month after PCI.
*DAPT: Dual Anti-Platelet Therapy
^SAPT: Single Anti-Platelet Therapy
XIENCE™ Stent remains the ONLY DES with the shortest DAPT indication, as short as 28 days.12
References
- Zanchin C, et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675. Serruys P, et al. N Engl J Med. 2010;363:136-146. Shiomi H, et al. JACC Cardiovasc Interv. 2019;12:637-647. Kufner S, et al. Circulation. 2019:139(3):325-333. Palmerini T, et al. Lancet. 2013;379:1393-1402. Bangalore S, et al. Circulation. 2012;125:2873-2891. Bangalore S, et al. Circ Cardiovasc Interv. 2013;6(6):378-390. Pilgrim T, et al. Lancet. 2014;384:2111-2122. Pilgrim T, et al. Lancet. 2018;392:737-746.
- Valgimigli M, et al. TCT Connect 2020 – XIENCE 28. Mehran R, et al. TCT Connect 2020 – XIENCE 90.
- Généreux P, et al. Circ Cardiovasc Interv. 2015;8(5):e00136. Natsuaki M, et al. Cardiovasc Interv Ther. 2016;31:196–209. Watanabe H, et al. JAMA. 2019;321(24):2414-2427. Hahn J, et al. ACC 2019 – SMART CHOICE. Valgimigli M, et al. Circulation. 2012;125:2015-2026. Gilard M, et al. J Am Coll Cardiol. 2015;65:777-786. Hong SJ, et al. JACC Cardiovasc Interv. 2016;9:1438–1446. Gwon HC, et al. ACC 2011 – EXCELLENT. Mehran R, et al. TCT Connect 2020 – XIENCE 28/90. Natsuaki, M., et al. ESC 2023 - STOPDAPT-3.
- Mehran R, et al. TCT Connect 2020 – XIENCE 28/90. Watanabe H, et al. JAMA. 2019;321(24):2414-2427. Hahn J, et al. ACC 2019 – SMART CHOICE. Varenne O, et al. Lancet. 2018.391:41-50 – SENIOR. Kirtane A, et al. TCT 2019 – EVOLVE Short DAPT. Kedhi E, et al. PCT eCourse 2020 – OnyxOne; Postma W, et al. Cather Cardiovasc Interv. 2020;95:706-710 – DAPT-STEMI. Valgimigli, M., et al. NEJM. 2021;10.1056
- Mehran R, et al. TCT Connect – 2020 XIENCE 28/90. Watanabe H, et al. JAMA. 2019;321(24):2414-2427. Natsuaki M, et al. Cardiovasc Interv Ther. 2016;31:196–209.
- Mehran R, et al. TCT Connect 2020 – XIENCE 28/90.
- Valgimigli M, et al. TCT Connect 2020 – XIENCE 28. Jinnouchi H, et al. J Am Coll Cardiol. 2019;74(Suppl B):B290 – TCT-291.
- Jinnouchi H, et al. J Am Coll Cardiol. 2019;74(Suppl B):B290 – TCT-291.
- Natsuaki M, et al. Cardiovasc Interv Ther. 2016;31:196–209.
- Watanabe H, et al. JAMA. 2019;321(24):2414-2427.
- Natsuaki, M., et al. AHA. 2023;1:49:00.
- XIENCE Skypoint™ Stent - Instructions For Use (IFU). Refer to IFU for additional information. Shortest as compared to commercially available competitor DES products. Reference: Competitor product IFUs: Synergy‡, Resolute Onyx‡, BioFreedom‡, Ultimaster‡ and Orsiro‡.
MAT-2101787 v4.0
Disclaimer
You are about to enter an Abbott country- or region-specific website.
Please be aware that the website you have requested is intended for the residents of a particular country or countries, as noted on that site. As a result, the site may contain information on pharmaceuticals, medical devices and other products or uses of those products that are not approved in other countries or regions
Do you wish to continue and enter this website?
MAT-2305078 v1.0

