Broadest Expansion Range Among XIENCE™ Stents2
XIENCE Skypoint™ Stent has improved maximum expansion compared to previous generations, so interventional cardiologists (ICs) can treat a broader range of vessels.

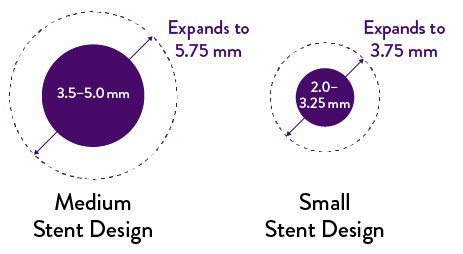
Expansion up to 5.75 mm
For ICs encountering larger vessels, this stent provides significant stent expansion: up to 5.75 mm when deploying 3.5, 4.0, 4.5, and 5.0 mm stents.
Smaller stents—2.0, 2.25, 2.5, 2.75, 3.0, 3.25 mm diameters—continue to expand to 3.75 mm.2
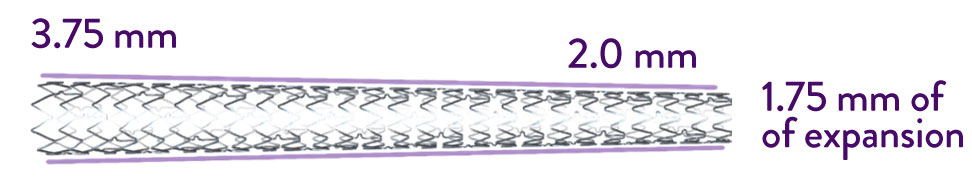
Even with greater expansion options, XIENCE™ Stents exhibit minimal shortening.3 This can help to ensure accurate stent placement and reduce the risk of geographic miss, which has been reported in over 30% of percutaneous coronary interventions (PCI).4

XIENCE Skypoint™ Stent for Tapered Lesions
XIENCE Skypoint™ Stent exhibits excellent expansion and apposition for a wide range of vessel sizes while also delivering optimal patient outcomes.5 Moreover, treating long, tapered lesions is practical with a single stent, especially since the XIENCE Skypoint™ Stent is available in a 48 mm length.6

Large Tapered Vessels: XIENCE Skypoint™ Stent provides a large expansion range designed to help treat patients with large tapered lesions—with a single stent.6
Small Tapered Vessels: Similarly, choosing a smaller diameter XIENCE Skypoint™ Stent allows ICs to treat a small tapered vessel, from 2.0 mm to 3.75 mm.6
Better Longitudinal Strength
The XIENCE™ family of stents are built on the MULTI-LINK platform. Its 3 links per ring connect the peaks of one ring to the valleys of the adjacent ring. This prevents struts from either compressing together or stretching apart.
With the MULTI-LINK stent platform, XIENCE™ Stents have better longitudinal strength, compared to other DES.7 This reduces the possibility of displacement or deformation for XIENCE Skypoint™ Stents.
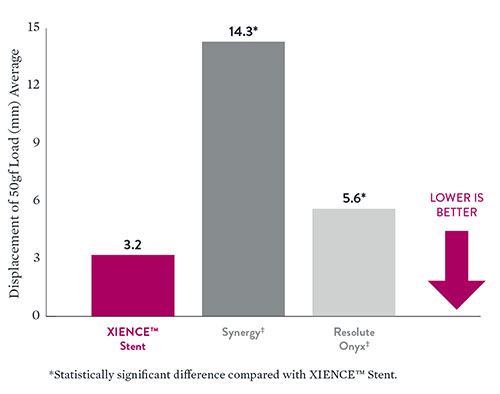
Compression tests reveal significantly improved longitudinal strength with XIENCE™ Stents compared to Synergy‡ and Resolute Onyx‡ DES.
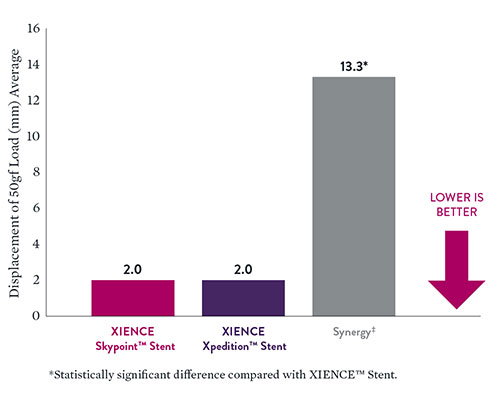
XIENCE Skypoint™ Stent Comparison: 3.0 mm x 48 mm8
Compression tests of 3.0 mm x 48 mm stents reveal excellent longitudinal strength with XIENCE Skypoint™ Stent—similar to XIENCE Xpedition™ Stent but significantly better than Synergy‡. So even when long lesions require PCI, the longitudinal strength of XIENCE Skypoint™ Stent sets it apart from other DES.
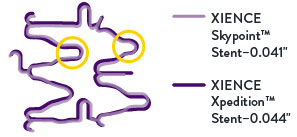
Lower Crossing Profile on 48 mm Length9
For long lesions, the 48 mm XIENCE Skypoint™ Stent, compared to the XIENCE Xpedition™ Stent, is designed with narrower crests and tighter nesting. These features allow for a lower crossing profile of just 0.041".
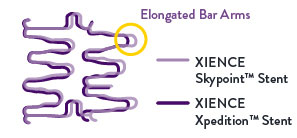
Better Expansion on 48 mm Length10
For long lesions, the 48 mm XIENCE Skypoint™ Stent’s design enhancements allow for improved expansion. Compared to the XIENCE Xpedition™ Stent, XIENCE Skypoint™ Stent is designed with elongated bar arms—offering better post-dilation (5.75 mm) expansion.
Together, all of these features lead to trusted patient outcomes, regardless of the diameter and length of the XIENCE Skypoint™ Stent deployed.
References
- Zanchin C, et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675. Serruys P, et al. N Engl J Med. 2010;363:136-146. Shiomi H, et al. JACC Cardiovasc Interv. 2019;12:637-647. Kufner S, et al. Circulation. 2019:139(3):325-333. Palmerini T, et al. Lancet. 2013;379:1393-1402. Bangalore S, et al. Circulation. 2012;125:2873-2891. Bangalore S, et al. Circ Cardiovasc Interv. 2013;6(6):378-390. Pilgrim T, et al. Lancet. 2014;384:2111-2122. Pilgrim T, et al. Lancet. 2018;392:737-746. Data on file at Abbott.
- XIENCE Skypoint™ Stent Instructions For Use (IFU). Refer to IFU for additional information. XIENCE Skypoint™ Stent compared with XIENCE Sierra™ Stent, 3.5 x 18 mm stent expanded to 5.75 mm. Acceptance criterion allows 10% as maximum.
- Data on file at Abbott. Comparison is between XIENCE Skypoint™ Stent, Synergy‡ and Resolute Onyx‡—3.5 x 18 mm or 20 mm stents tested and expanded to maximum labelled expansion limit.
- Calvert PA. Catheter Cardiovasc Interv. 2016;88(3):340-347.
- Sudhir K, et al. ISRN Cardiol. 2013:748736.
- XIENCE Skypoint™ Instructions for Use (IFU). Refer to IFU for additional information.
- Data on file at Abbott. XIENCE Sierra™ Stent (5.0 x 33 mm) n=5, SYNERGY‡ (5.0 x 32 mm) n=5, Resolute Onyx‡ (5.0 x 33 mm) n=5.
- Data on file at Abbott. XIENCE Skypoint™ Stent (3.0 x 48 mm) n=4, XIENCE Xpedition™ Stent (3.0 x 48 mm) n=5, SYNERGY‡ (3.0 x 48 mm) n=3.
- Data on file at Abbott. Average stent profile: XIENCE Skypoint™ Stent 3.0 x 48 mm compared with XIENCE Xpedition™ Stent 3.0 x 48 mm. Note: Small stent designs include 2.5 x 48 mm, 2.75 x 48 mm and 3.0 x 48 mm sizes
- Data on file at Abbott. Note: Elongated bar arms is for medium stent design (9-crest design), 3.5 mm and 4.00 mm.
MAT-2107572 v3.0
