Market-Leading Everolimus
The market-leading choice for XIENCE™ Stent is everolimus, which is a proven therapeutic drug.²
An ideal drug for DES has these characteristics:
- Safe over the long term
- Stable over time
- Compatible with polymer
- Cytostatic and anti-inflammatory
- Effective at varying doses for a wide therapeutic window
The everolimus drug provides all these features.
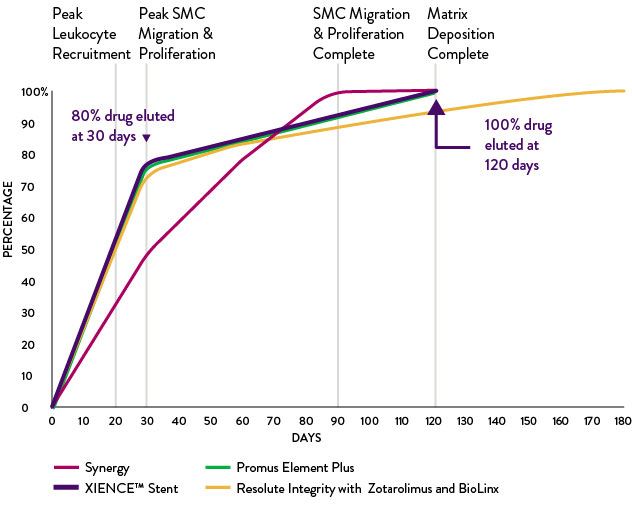
Everolimus Elution Rate Matches Restenosis Cascade for Optimal Endothelization Rate3
The release of everolimus aligns with the vessel's restenosis cascade. This, in turn, maximizes the drug's efficacy against restenosis and minimizes the drug's effect of re-endothelialization, which facilitates vessel restoration and function.
Everolimus Elution Correlates to Restenosis Cascade
XIENCE™ Stent Uses Low Dose of Everolimus3,4
Everolimus is a well established anti-proliferative drug. The concentration of everolimus used with XIENCE™ Stent is low, at 100 µg/cm². These histological images below show healthy, fully healed vessels at a concentration that is 8x the dose used on the XIENCE™ stent. Images were gathered using a pre-clinical animal model.
Everolimus Safety at 8x Drug Dose

References
- Zanchin C, et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675. Serruys P, et al. N Engl J Med. 2010;363:136-146. Shiomi H, et al. JACC Cardiovasc Interv. 2019;12:637-647. Kufner S, et al. Circulation. 2019:139(3):325-333. Palmerini T, et al. Lancet. 2013;379:1393-1402. Bangalore S, et al. Circulation. 2012;125:2873-2891. Bangalore S, et al. Circ Cardiovasc Interv. 2013;6(6):378-390. Pilgrim T, et al. Lancet. 2014;384:2111-2122. Pilgrim T, et al. Lancet. 2018;392:737-746. Data on file at Abbott.
- XIENCE Skypoint™ Stent Instruction For Use (IFU). Refer to IFU for additional information.
- Perkins L, et al. J Interven Cardiol. 2009;22:S28-S40; Bennett J, Biologics: Targets and Therapy. 2013;7:149-159; Meredith I et al. J Am Cardiol Intv. 2009;2:977-985.
- Data on file at Abbott.
MAT-2101779 v2.0
