XIENCE™ Stent Clinical Outcomes
Stent choice matters. And a wealth of clinical evidence supports the safety of the XIENCE™ Stent, which is why experts consider XIENCE™ Stent to be the gold standard among DES.1
In addition, reproducible procedural outcomes are a proven quality of XIENCE™ DES performance,2 delivering consistent patient safety with low complication rates3
When choosing XIENCE™ Stent, interventional cardiologists (ICs) can know that the optimal outcomes they achieved in the cath lab will persist far into the future—not only treating stenosed vessels but enabling patients to have a better quality of life.4
There’s a Whole World of Evidence to Support the XIENCE™ Stent
XIENCE™ Stent is the world's leading DES, with over 20 million implants.5




Substantial clinical data continue to provide evidence that XIENCE™ DES can effectively treat a broad spectrum of patients.1 Given the best in class deliverability and the expanded treatment options of XIENCE Skypoint™ Stent,7 it is an excellent choice for complex patients.
Key XIENCE™ Stent Clinical Trials
XIENCE 28 & XIENCE 90 Data
XIENCE™ Stent—with either 1-month or 3-month dual antiplatelet therapy (DAPT)—reduced severe bleeding with no increase in ischemic events.8
Short DAPT: Reduced Severe Bleeding8
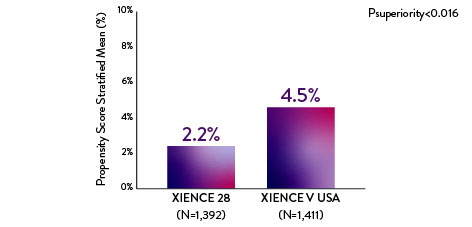
XIENCE 28: BARC 3-5 BLEEDING
Between 1 and 6 months
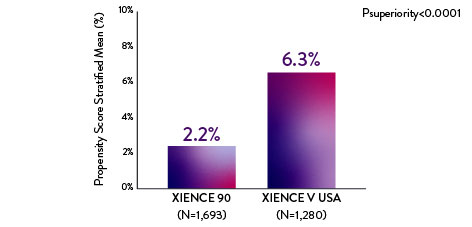
XIENCE 90: BARC 3-5 BLEEDING
Between 3 and 12 months
Note: PS stratified analysis for BARC 3-5 bleeding was not pre-specified.
Note: BARC 2-5 was a powered secondary endpoint.
Short DAPT: No Increase in Ischemic Events in High Bleeding Risk (HBR) Patients8
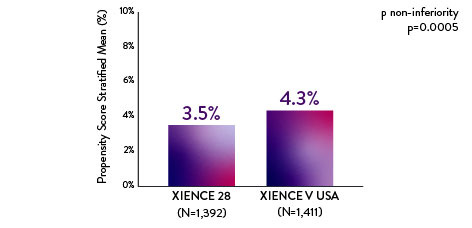
XIENCE 28: 1-month DAPT in HBR Patients
XIENCE 28: All Deaths or MI
Between 1 and 6 months
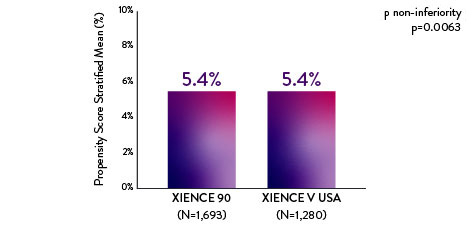
XIENCE 90: 3-month DAPT in HBR Patients
XIENCE 90: All Deaths or MI
Between 3 and 12 months
CARDIOBASE Registry: XIENCE™ Stent vs Synergy‡
XIENCE™ Stent outperforms Synergy‡ with short-term safety outcomes in real-world patients.9
Short-Term Data with XIENCE™ Stent and Synergy‡
XIENCE™ Stent: Significantly Lower Acute Definite Stent Thrombosis vs Synergyǂ9
75% Less acute definite stent thrombosis with XIENCE™ Stent System compared to Synergyǂ
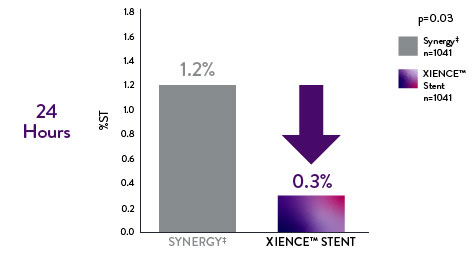
XIENCE™ Stent: Significantly Lower Target Lesion Revascularization (TLR) at 1 Month vs Synergyǂ9
Suggests favorable economic benefits* for XIENCE Stent vs. Synergyǂ in clinical practice
*Driven by reduction in additional procedures and hospitalizations
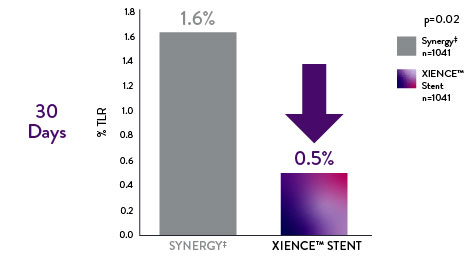
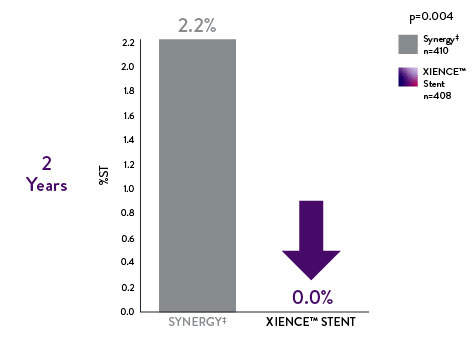
IDEAL-LM Data: Long-Term Complex Patient Data on XIENCE™ Stent vs Synergy‡
In the left main subset of patients, the IDEAL-LM findings also reveal that the XIENCE™ Stent outperforms Synergy‡ at 2 years10
Left Main Patients: XIENCE™ Stent Shows Significantly Lower Long-Term Definite Stent Thrombosis vs Synergyǂ10
XIENCE Stent
0% Stent Thrombosis
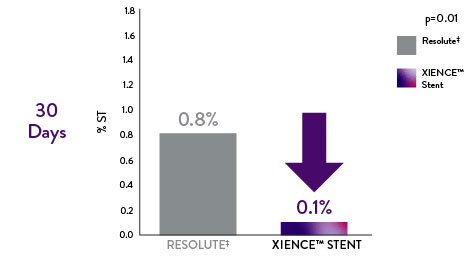
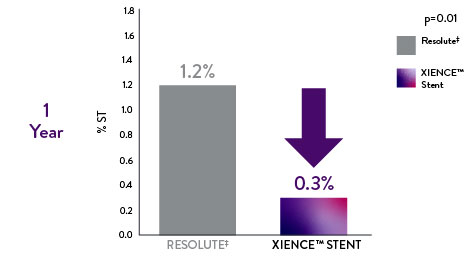
RESOLUTE All-Comers (RAC) Randomized Trial: XIENCE™ Stent vs Resolute‡
Safety data from RAC real-world patients also reveal that XIENCE™ Stent outperforms Resolute.‡11
XIENCE™ Stent: Significantly Lower Early Definite Stent Thrombosis vs Resoluteǂ11
88% less definite stent thrombosis with XIENCE™ Stent compared to Resoluteǂ
XIENCE™ Stent: Significantly Lower Late Definite Stent Thrombosis vs Resoluteǂ11
75% less definite stent thrombosis with XIENCE™ Stent compared to Resoluteǂ
Note: Primary endpoint for non-inferiority was met by Resoluteǂ compared with XIENCE™ Stent. The primary endpoint of target lesion failure (TLF) was a composite of cardiac death, myocardial infarction, and clinically indicated target lesion revascularization at 12 months.
References:
- Zanchin C, et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675. Serruys P, et al. N Engl J Med. 2010;363:136-146. Shiomi H, et al. JACC Cardiovasc Interv. 2019;12:637-647. Kufner S, et al. Circulation. 2019:139(3):325-333. Palmerini T, et al. Lancet. 2013;379:1393-1402. Bangalore S, et al. Circulation. 2012;125:2873-2891. Bangalore S, et al. Circ Cardiovasc Interv. 2013;6(6):378-390. Pilgrim T, et al. Lancet. 2014;384:2111-2122. Pilgrim T, et al. Lancet. 2018;392:737-746. Data on file at Abbott.
- Saito S, et al. Eur Heart J. 2014;35:2021-2031. Saito S, et al. EuroIntervention. 2019;15(11):e1006-e1013. Natsuaki M, et al. J Am Coll Cardiol. 2013;62:181-190. Stone G, et al. Lancet 2018;392:1530-1540.
- Zanchin C, et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675. Serruys P, et al. N Engl J Med. 2010;363:136-146. Shiomi H, et al. JACC Cardiovasc Interv. 2019;12:637-647. Kufner S, et al. Circulation. 2019:139(3):325-333.
- Baron SJ, et al. J Am Coll Cardiol. 2017;70:3113-3122.
- Data on file at Abbott. 20 million implants is based on DES data through Q4 2022.
- Kufner S, et al. Circulation. 2019:139(3):325-333.
- Data on file at Abbott. XIENCE Sierra™ 3.0 x 18 mm, n=5; Synergy‡ 3.0 x 20 mm, n=5; Resolute‡ Onyx 3.0 x 18 mm, n=5. XIENCE Skypoint™ Stent Instructions for Use (IFU). Refer to IFU for additional information.
- Mehran R, et al. TCT Connect 2020, XIENCE 28/90.
- Zanchin C. et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675.
- Van Geuns RJ, et al. TCT 2019, IDEAL-LM.
- Serruys P, et al. N Engl J Med. 2010;363:136-146.
MAT-2101785 v2.0
