XIENCE™ Stent: Protecting Patients with Short DAPT Needs
Dual antiplatelet therapy (DAPT) duration often depends on the patient—and it may depend on the stent as well.
Stent Design for Short-Term DAPT in HBR Patients: What is Required?
HBR Clinical Data and Future Trials
XIENCE™ Stent Has the Largest Body of DAPT Patient Evidence3
XIENCE™ Stent is the only DES that is supported by 1 and 3 months of DAPT data. XIENCE™ Stent is the only DES with results showing 1-month DAPT data and 3-month DAPT data for both Aspirin and P2Y12 inhibitor monotherapy—including data on patients at high bleeding risk (HBR).4
Among all 3 categories outlined above—aspirin monotherapy, P2Y12 inhibitor monotherapy and HBR patients—XIENCE™ Stent data revealed very low rates of stent thrombosis (ST) at 12 months.5
≤ 0.3% Definite ST At 1 Year5
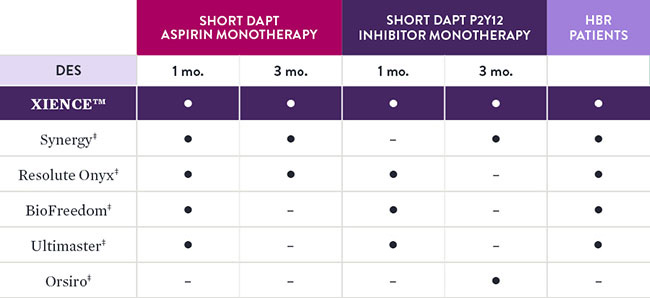
Comparison of Short DAPT Studies by Stent Type4
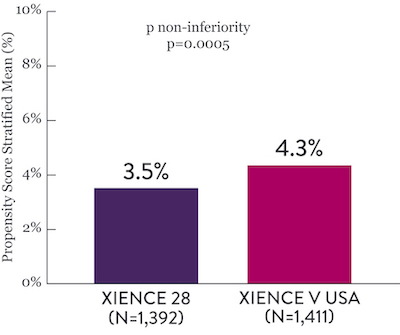
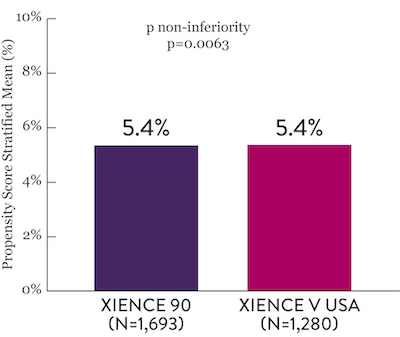
XIENCE 28 and XIENCE 90 Study Results6
XIENCE™ Stent Short DAPT: Ischemic Events
Among HBR patients, XIENCE™ Stent with 1-month or 3-month DAPT reduced severe bleeding with no increase in ischemic events, including myocardial infarction (MI) and all death.6
XIENCE 28: 1-month DAPT in HBR Patients
XIENCE 28: All Death or MI
Between 1 and 6 months
XIENCE 90: 3-month DAPT in HBR Patients
XIENCE 90: All Death or MI
Between 3 and 12 months
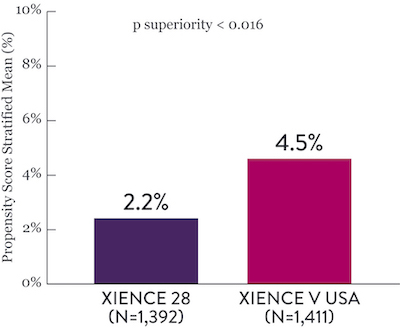
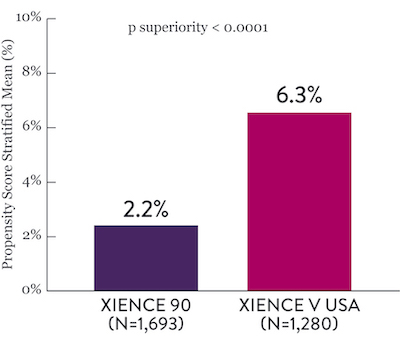
XIENCE™ Stent Short DAPT: Reduced Severe Bleeding
In the same HBR population, XIENCE™ Stent with 1-month or 3-month DAPT reduced severe bleeding with no increase in ischemic events.6,*
XIENCE 28: BARC 3-5 Bleeding
Between 1 and 6 months
XIENCE 90: BARC 3-5 Bleeding
Between 3 and 12 months
*Propensity score stratified analysis for BARC 3-5 bleeding was not pre-specified. BARC 2-5 was a powered secondary endpoint. In both studies, for BARC 2-5, XIENCE™ Stent showed numerically lower bleeding rate for 1-month or 3-month DAPT versus 6-month DAPT or 12-month DAPT, respectively.
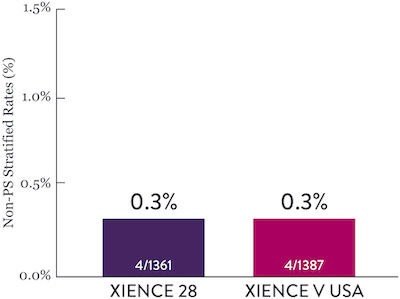
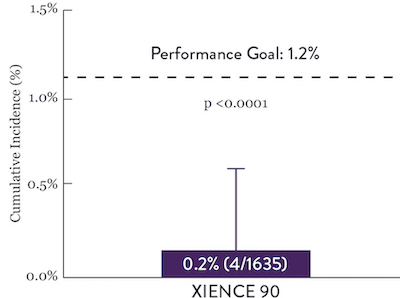
XIENCE™ Stent Short DAPT: Continued Low Stent Thrombosis
XIENCE™ Stent has shown, in a large number of trials, extremely low rates of ST and it has shown to have better antithrombotic properties in preclinical studies..7 Rates of ST in Short DAPT conditions are consistent with previously reported rates with longer DAPT. XIENCE™ Stent showed an ST rate of 0.3% (1-month DAPT) and 0.2% (3-month DAPT).6
XIENCE 28: Stent Thrombosis
Between 1 and 6 months
ARC : Definite/Probable ST
XIENCE 90: Stent Thrombosis
Between 3 and 12 months
ARC : Definite/Probable ST
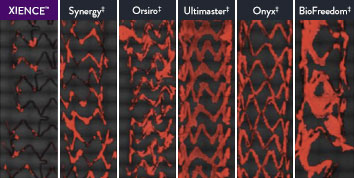
XIENCE™ Stent Shows Strong Anti-Thrombotic Properties: Suited for Short DAPT
The XIENCE™ Stent has shown significantly less thrombus formation in preclinical studies versus other DES on the market. As shown in the study findings, XIENCE™ Stent reveals significantly less (p < 0.01) platelet adhesion—shown in red in the confocal microscopy images—than other DES, and platelet adhesion is an important factor in stent thrombosis.*8 These findings suggest that this stent choice “may be ideally suited for very short-term DAPT.”8
*Ex Vivo Swine Shunt Model.
STOPDAPT Studies: 1-Month DAPT and 3-Month DAPT in All-Comers Population9,10
STOPDAPT9 and STOPDAPT 210 were prospective trials of the XIENCE™ Stent that studied DAPT cessation at 3 months and 1 month, respectively
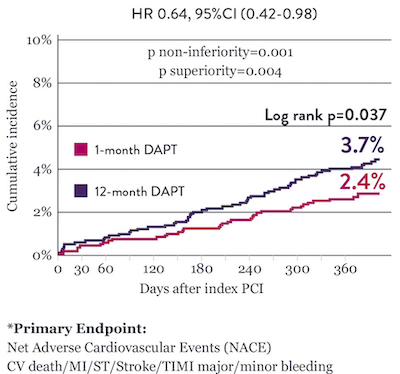
STOPDAPT 2 Trial: 1-Month DAPT Superior to 12-Month DAPT10
The STOPDAPT 2 trial revealed that 1-month DAPT demonstrated superior safety over 12-month DAPT for the primary endpoint of net adverse cardiovascular events (NACE). NACE included cardiovascular death, myocardial infarction (MI), definite ST, stroke, or thrombolysis in MI (TIMI) major/minor bleeding. All of the 3,009 patients in this randomized, controlled trial were treated with XIENCE™ Stent.10
Significantly Lower NACE* with 1-Month DAPT
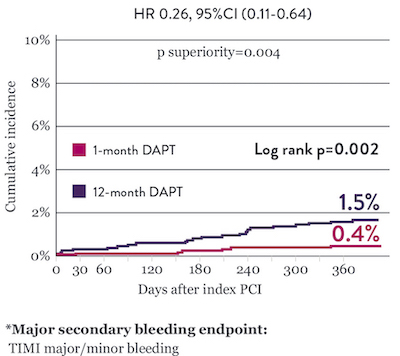
Significantly Lower Bleeding Events* with 1‑Month DAPT
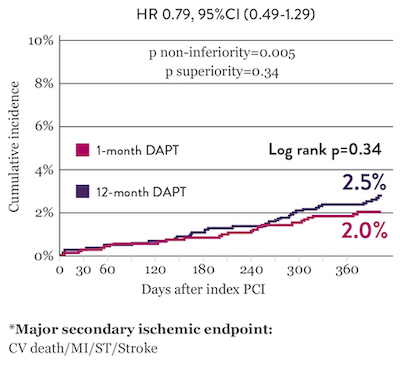
Comparable Ischemic Event Rates* with 1‑Month DAPT
“Stopping DAPT at 3 months in selected patients after [XIENCE™ Stent] implantation was at least as safe as the prolonged DAPT regimen adopted in the historical control group.”
— Masahiro Natsuaki, MD, STOPDAPT Trial9


STOPDAPT 2 Trial Design and Randomization10
Short 1-Month DAPT
- 0 to 1-month: Aspirin + P2Y12
- After 1-month: Clopidogrel monotherapy

12-Month DAPT
- 0 to 1-month: Aspirin + P2Y12
- 1 to 12-month: Aspirin + Clopidogrel
- 12 to 60-month: Aspirin monotherapy

- Successful PCI using CoCr everolimus-eluting stent: XIENCE™
- Eligible for DAPT (aspirin/P2Y12 receptor blocker) for 1 year

- Patients who need oral anticoagulants
- History of intracranial bleeding
- Major in-hospital complications (MI/stroke/major bleeding)

STOPDAPT Study: XIENCE™ Stent with 3-Month DAPT Is Feasible9
STOPDAPT9 was the first prospective trial to study DAPT cessation at 3 months after implantation. Among other 1-year outcomes, the XIENCE™ Stent rate of stent thrombosis was 0.0%.
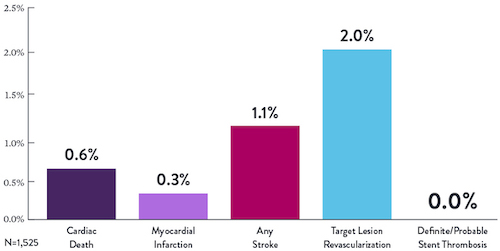
“It was noteworthy that no definite or probable stent thrombosis occurred in [XIENCE™ Stent] patients enrolled in STOPDAPT.”
— Masahiro Natsuaki, MD, STOPDAPT Trial9
STOPDAPT-3 Trial Design and Randomization11
- PCI with planned exclusive use of CoCr-EES (XIENCE)
- ACS presentation or ARC-HBR
- Eligible for DAPT (Aspirin/P2Y12inhibitor) for 1 month

Study design and Randomization
Group 1:
0 to 1-month: Aspirin + P2Y12 (Prasugrel)
After 1-month: Clopidogrel monotherapy
Group 2:
0 to 1-month: P2Y12 (Prasugrel)
After 1-month: Clopidogrel monotherapy
STOPDAPT-3 Trial 11 was designed to explore 0-month DAPT* (SAPT˄ using only P2Y12 inhibitor) for ACS and HBR patients.
Though the results are comparable for both bleeding and ischemic events for DAPT and SAPT arms, the study did not meet its endpoint and concluded to use DAPT for 1 month after PCI.
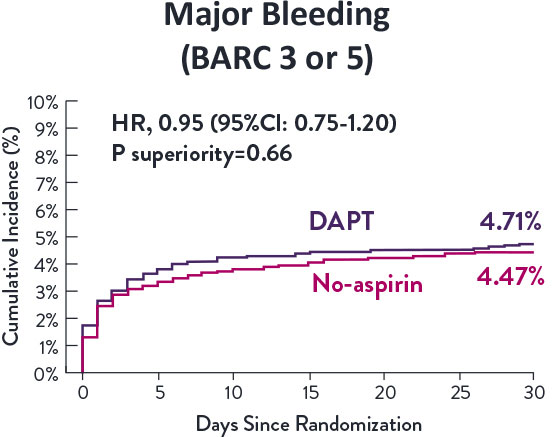
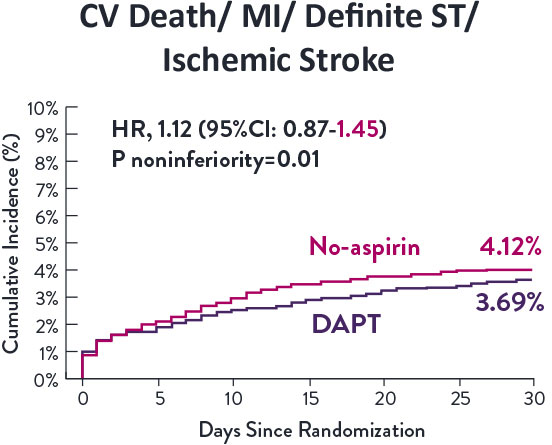
XIENCE™ Stent remains the ONLY DES with the shortest DAPT indication, as short as 28 days.12
References
- Zanchin C, et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675. Serruys P, et al. N Engl J Med. 2010;363:136-146. Shiomi H, et al. JACC Cardiovasc Interv. 2019;12:637-647. Kufner S, et al. Circulation. 2019:139(3):325-333. Palmerini T, et al. Lancet. 2013;379:1393-1402. Bangalore S, et al. Circulation. 2012;125:2873-2891. Bangalore S, et al. Circ Cardiovasc Interv. 2013;6(6):378-390. Pilgrim T, et al. Lancet. 2014;384:2111-2122. Pilgrim T, et al. Lancet. 2018;392:737-746.
- Valgimigli M, et al. TCT Connect 2020 – XIENCE 28. Mehran R, et al. TCT Connect 2020 – XIENCE 90.
- Généreux P, et al. Circ Cardiovasc Interv. 2015;8(5):e00136. Natsuaki M, et al. Cardiovasc Interv Ther. 2016;31:196–209. Watanabe H, et al. JAMA. 2019;321(24):2414-2427. Hahn J, et al. ACC 2019 – SMART CHOICE. Valgimigli M, et al. Circulation. 2012;125:2015-2026. Gilard M, et al. J Am Coll Cardiol. 2015;65:777-786. Hong SJ, et al. JACC Cardiovasc Interv. 2016;9:1438–1446. Gwon HC, et al. ACC 2011 – EXCELLENT. Mehran R, et al. TCT Connect 2020 – XIENCE 28/90. Natsuaki, M., et al. ESC 2023 - STOPDAPT-3.
- Mehran R, et al. TCT Connect 2020 – XIENCE 28/90. Watanabe H, et al. JAMA. 2019;321(24):2414-2427. Hahn J, et al. ACC 2019 – SMART CHOICE. Varenne O, et al. Lancet. 2018.391:41-50 – SENIOR. Kirtane A, et al. TCT 2019 – EVOLVE Short DAPT. Kedhi E, et al. PCT eCourse 2020 – OnyxOne; Postma W, et al. Cather Cardiovasc Interv. 2020;95:706-710 – DAPT-STEMI. Valgimigli, M., et al. NEJM. 2021;10.1056
- Mehran R, et al. TCT Connect – 2020 XIENCE 28/90. Watanabe H, et al. JAMA. 2019;321(24):2414-2427. Natsuaki M, et al. Cardiovasc Interv Ther. 2016;31:196–209.
- Mehran R, et al. TCT Connect 2020 – XIENCE 28/90.
- Valgimigli M, et al. TCT Connect 2020 – XIENCE 28. Jinnouchi H, et al. J Am Coll Cardiol. 2019;74(Suppl B):B290 – TCT-291.
- Jinnouchi H, et al. J Am Coll Cardiol. 2019;74(Suppl B):B290 – TCT-291.
- Natsuaki M, et al. Cardiovasc Interv Ther. 2016;31:196–209.
- Watanabe H, et al. JAMA. 2019;321(24):2414-2427.
- Natsuaki, M., et al. AHA. 2023;1:49:00.
- XIENCE Skypoint™ Stent - Instructions For Use (IFU). Refer to IFU for additional information. Shortest as compared to commercially available competitor DES products. Reference: Competitor product IFUs: Synergy‡, Resolute Onyx‡, BioFreedom‡, Ultimaster‡ and Orsiro‡.
*DAPT: Dual Anti-Platelet Therapy
^SAPT: Single Anti-Platelet Therapy
MAT-2404534 v1.0
XIENCE Skypoint™, XIENCE Sierra™, XIENCE Alpine™ (XIENCE™ Family) Everolimus Eluting Coronary Stent Systems

Indications
Applies to XIENCE Skypoint™ Stent Systems:
- Indicated for improving coronary artery luminal diameter in patients, including those with diabetes mellitus, with symptomatic heart disease due to de novo native coronary artery lesions ≤ 44 mm in length with reference vessel diameters of ≥ 2.25 mm to ≤ 5.25 mm and for high bleeding risk patients with coronary arteries lesions ≤ 32 mm in length with a reference vessel diameter of ≥ 2.25 mm and ≤ 5.25 mm.
- Treating de novo chronic total coronary occlusions.
Applies to XIENCE Sierra™ and XIENCE Alpine™ Stent Systems:
- Indicated for improving coronary artery luminal diameter in patients, including those at high risk for bleeding and those with diabetes mellitus, with symptomatic heart disease due to de novo native coronary artery lesions (length ≤ 32 mm) with reference vessel diameters of ≥ 2.25 mm to ≤ 4.25 mm.
- Treating de novo chronic total coronary occlusions.
Contraindications
The XIENCE Skypoint™, XIENCE Sierra™ and XIENCE Alpine™ Stent Systems are contraindicated for use in:
- Patients who cannot tolerate, including allergy or hypersensitivity to, procedural anticoagulation or the post-procedural antiplatelet regimen.
- Patients with hypersensitivity or contraindication to everolimus or structurally-related compounds, or known hypersensitivity to stent components (cobalt, chromium, nickel, tungsten, methacrylic polymer, fluoropolymer), or with contrast hypersensitivity.
Warnings
- Each stent and the delivery system are for single use only. Do not reuse, reprocess, or resterilize. Note the product “Use by” date on the package. Reuse, reprocessing, or resterilization may compromise the structural integrity of the device and / or delivery system and / or lead to device failure, which may result in patient injury, illness, or death. Reuse, reprocessing, or resterilization may also create a risk of contamination of the device and / or cause patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device and / or delivery system may lead to injury, illness, or death of the patient.
- It is not recommended to treat patients having a lesion that prevents complete inflation of an angioplasty balloon.
- Antiplatelet therapy should be administered post-procedure.
- This product should not be used in patients who are not likely to comply with the recommended antiplatelet therapy.
- Judicious selection of patients is necessary, since the use of this device carries the associated risk of stent thrombosis, vascular complications, and/or bleeding events.
- The XIENCE Skypoint™, XIENCE Sierra™ and XIENCE Alpine™ Stent Systems are coated with an everolimus and polymer coating at the full implant stent length. The distal and intermediate portions of the device, the tip, and tapers of the balloon are coated with HYDROCOAT™ Hydrophilic Coating.
Failure to abide by the warnings in this labeling might result in damage to the device coating, which may necessitate intervention or result in serious adverse events.
Precautions
- Implantation of the stent should be performed only by the physicians who have received appropriate training.
- Stent placement should be performed at centers where emergency coronary artery bypass graft surgery (CABG) can be readily performed.
- When the XIENCE Skypoint™, XIENCE Sierra™ and XIENCE Alpine™ Stent Systems are used outside the specified Indications for Use, patient outcomes may differ from the results observed in the SPIRIT family of clinical trials. Compared to use within the specified indications for use, the use of the XIENCE Skypoint™, XIENCE Sierra™ and XIENCE Alpine™ Stent Systems in patients and lesions outside of the labeled indications, including more tortuous anatomy, may have an increased risk of adverse events, including stent thrombosis, stent embolization, MI, or death.
- The extent of the patient’s exposure to drug and polymer is directly related to the number of stents implanted. See Instructions for Use for current data on multiple stent implantation.
- Safety and effectiveness of the XIENCE Skypoint™, XIENCE Sierra™ and XIENCE Alpine™ Stent Systems have not been established for subject populations with the following clinical settings:
- Patients with prior brachytherapy of the target lesion or the use of brachytherapy for treated site restenosis.
- Conjunctive use of the XIENCE Skypoint™, XIENCE Sierra™ and XIENCE Alpine™ Stent Systems with either mechanical atherectomy devices or laser angioplasty catheters.
- Women who are pregnant or lactating, men intending to father children, pediatric.
- Unresolved vessel thrombus at the lesion site, coronary artery reference vessel diameters < 2.25 mm or > 4.25 mm, for XIENCE Skypoint™ < 2.25 mm or > 5.25 mm, or lesion lengths >44 mm, lesions located in saphenous vein grafts, lesions located in unprotected left main coronary artery, ostial lesions, or lesions located at a bifurcation or previously stented lesions, diffuse disease or poor flow (TIMI < 1) distal to the identified lesions, excessive tortuosity proximal to or within the lesion, recent Acute Myocardial Infarction (AMI) or evidence of thrombus in target vessel, multivessel disease, and in-stent restenosis.
- Formal drug interaction studies have not been performed with the XIENCE Skypoint™, XIENCE Sierra™ or XIENCE Alpine™ Stent Systems because of limited exposure to everolimus eluted from XIENCE Skypoint™, XIENCE Sierra™ and XIENCE Alpine™ Stent Systems.
- Everolimus, the active ingredient in the stents, is an immunosuppressive agent. Consideration should be given to patients taking other immunosuppressive agents or who are at risk for immune suppression.
- Oral everolimus use in renal transplant and advanced renal cell carcinoma patients was associated with increased serum cholesterol and triglyceride levels, which in some cases required treatment.
- Nonclinical testing has demonstrated that the XIENCE Sierra™ and XIENCE Alpine™ Stent Systems in single and in overlapped configurations up to 71 mm, for XIENCE Skypoint™ 91 mm in length, are MR Conditional. See Instructions for Use for detailed scanning conditions
Potential Adverse Events
Adverse events that may be associated with PCI treatment procedures and the use of a stent in native coronary arteries include, but are not limited to, the following:
- Allergic reaction or hypersensitivity to latex, contrast agent anesthesia, device materials, and drug reactions to everolimus, anticoagulation, or antiplatelet drugs
- Vascular access complications which may require transfusion or vessel repair, including: Catheter site reactions, Bleeding, Arteriovenous fistula; pseudoaneurysm, aneurysm, dissection, perforation/rupture, Embolism, Peripheral nerve injury, Peripheral ischemia
- Coronary artery complications which may require additional intervention, including: Total occlusion or abrupt closure, Arteriovenous fistula, pseudoaneurysm, aneurysm, dissection. Perforation/rupture, Tissue prolapse/plaque shift, Embolism, Coronary or stent thrombosis, Stenosis or restenosis
- Pericardial complications which may require additional intervention, including: Cardiac tamponade, Pericardial effusion, Pericarditis.
- Cardiac arrhythmias
- Cardiac ischemic conditions (including myocardial ischemia, myocardial infarction (including acute), coronary artery spasm, and unstable or stable angina pectoris)
- Stroke/Cerebrovascular Accident (CVA) and Transient Ischemic Attack (TIA)
- System organ failures: Cardio-respiratory arrest, Cardiac failure, Cardiopulmonary failure, Renal Insufficiency/failure, Shock
- Bleeding
- Blood cell disorders
- Hypotension and/or hypertension
- Infection
- Nausea and vomiting
- Palpitations
- Dizziness
- Syncope
- Chest Pain
- Fever
- Pain
- Death
The risks described below include the anticipated adverse events relevant for the cardiac population referenced in the contraindications, warnings and precaution sections of the everolimus labels / SmPCs and / or observed at incidences ≥ 10% in clinical trials with oral everolimus for different indications. Please refer to the drug SmPCs and labels for more detailed information and less frequent adverse events.
- Abdominal pain
- Anemia
- Angioedema
- Arterial Thrombotic Events
- Bleeding and coagulopathy
- Constipation
- Cough
- Diabetes mellitus
- Diarrhea
- Dyspnea
- Embryo-fetal toxicity
- Erythema
- Erythroderma
- Headache
- Hepatic artery thrombosis
- Hepatic disorders
- Hypersensitivity to everolimus active substance, or to other rapamycin derivates
- Hypertension
- Infection (bacterial, fungal, viral or protozoan infections, including infections with opportunistic pathogens). Polyoma virus-associated nephropathy (PVAN), JC virus-associated progressive multiple leukoencephalopathy (PML), fatal infections and sepsis have been reported in patients treated with oral everolimus
- Kidney arterial and venous thrombosis
- Laboratory test alterations
- Lymphoma and skin cancer
- Male infertility
- Menstrual irregularities
- Nausea
- Nephrotoxicity
- Non-infectious pneumonitis
- Oral ulcerations
- Pain
- Pancreatitis
- Pericardial effusion
- Peripheral edema
- Pleural effusion
- Pneumonia
- Pyrexia
- Rash
- Renal Failure
- Upper respiratory tract infection
- Urinary tract infection
- Venous thromboembolism
- Vomiting
- Wound healing complications
There may be other potential adverse events that are unforeseen at this time.
MAT-2100879 v7.0



Stay Connected