XIENCE Sierra™ Stent
Even When Patients Are Complex—
Choosing a Stent Shouldn't Be
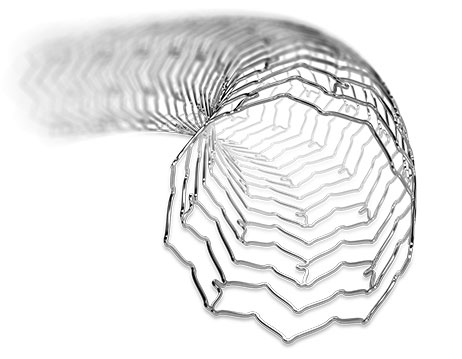
The design of XIENCE Sierra™ Stent is unlike that of other drug-eluting stents (DES). With its unique design, XIENCE Sierra™ Stent is proven to provide unparalleled patient outcomes1 during and far beyond the intervention. This in turn helps:
- Interventional cardiologists achieve enduring and positive patient outcomes2
- Patients achieve a better quality of life3
The stent design ensures scaffold integrity and stent stability with significantly greater longitudinal strength than other DES.4
XIENCE Sierra™ Stent provides:
Learn More About XIENCE Sierra™ Stent
You can view clinical data about XIENCE Sierra™ Stent. You can also order XIENCE Sierra™ Stent to treat a broad spectrum of patients and lesions.
References
- Zanchin C, et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675. Serruys P, et al. N Engl J Med. 2010;363:136-146. Shiomi H, et al. JACC Cardiovasc Interv. 2019;12:637-647. Kufner S, et al. Circulation. 2019:139(3):325-333. Palmerini T, et al. Lancet. 2013;379:1393-1402. Bangalore S, et al. Circulation. 2012;125:2873-2891. Bangalore S, et al. Circ Cardiovasc Interv. 2013;6(6):378-390. Pilgrim T, et al. Lancet. 2014;384:2111-2122. Pilgrim T, et al. Lancet. 2018;392:737-746.
- Shiomi H, et al. JACC Cardiovasc Interv. 2019;12:637-647. Kufner S, et al. Circulation. 2019:139(3):325-333.
- Baron SJ, et al. J Am Coll Cardiol. 2017;70:3113-3122.
- Data on file at Abbott. XIENCE Sierra™ Stent – 3.0 x 28 mm, n=5; SYNERGY‡ Stent – 3.0 x 28 mm, n=5; Resolute Onyx‡ Stent –3.0 x 28 mm, n=5; Orsiro‡ Stent System – 3.0 x 28 mm, n=5.
- Data on file at Abbott. XIENCE Sierra™ Stent – 3.0 x 18 mm, n=5; SYNERGY‡ Stent – 3.0 x 20 mm, n=5; Resolute Onyx‡ Stent –3.0 x 18 mm.
- XIENCE Sierra™ Stent Instructions for Use (IFU). XIENCE Alpine™ Stent IFU. Refer to IFUs for additional information.
MAT-2101792 v2.0
