Experience the OCT difference.
Request your Abbott Sales rep today.
What is Optical Coherence Tomography (OCT) Imaging?
Optical Coherence Tomography (OCT) is an intravascular imaging modality that uses near-infrared light to provide high-definition, cross-sectional and three-dimensional images of the vessel microstructure during percutaneous coronary intervention (PCI).
"These images provide additional information on the degree and characteristics of coronary artery disease compared to angiography which doesn’t delineate the composition of the coronary artery.1 With automated, highly accurate measurements, OCT can guide stent selection, placement, and deployment.1"
— Dr. Manuel M. Reyes, Interventional Cardiologist
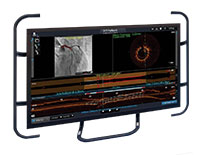
OCT Software User Interface
Ultreon™ Software is powered by artificial intelligence (AI) and automation. It has an improved and intuitive user-interface over the previous generation.*

Ultreon™ 1.0 Software User Interface. Pre-PCI pullback. Automatically displays degree and max arc of calcification.
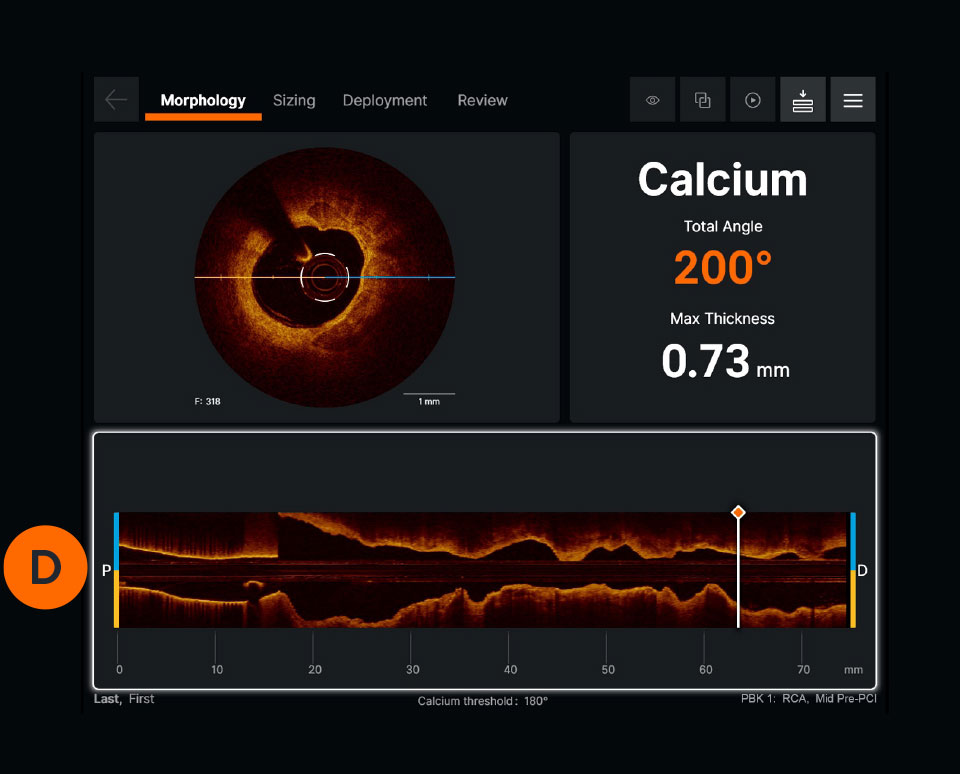
Ultreon™ 1.0 Software User Interface. Pre-PCI pullback. Automatically displays degree and max arc of calcification.
Previous generation AptiVue™ Software User Interface. Pre-PCI pullback.
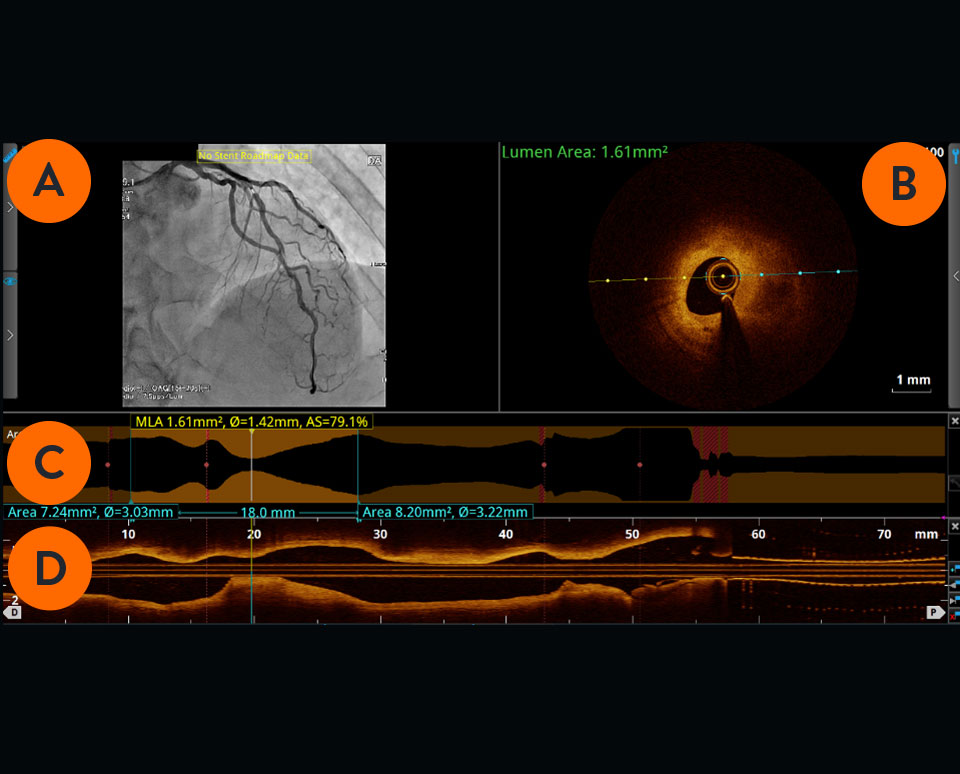
Angiogram co-registration denotes location of the cross-sectional OCT image.

Cross-sectional visualization is used to obtain detailed findings, such as structures in the lumen and in the various layers of the vessel wall.

The cross-sectional area of the vessel is visualized along the entire length of the examined vessel segment. It allows precise determination of the vessel diameter (mean reference diameter) and the length of the altered segment. The white marker is located at the position of the cross-section shown.

Viewed from left to right, the longitudinal section visualizes the scanned vessel segment from proximal to distal in Ultreon™ Software, and from distal to proximal in AptiVue™ Software.

How does OCT support PCI guidance?
- For pre-PCI guidance, OCT can be used to identify the culprit lesion, assess lesion morphology to select the right lesion preparation strategy, and characterize the stent landing zones to select precise length and diameter of balloons and stents.2
- For post-PCI guidance, OCT offers improved visualization of stent dissection, tissue protrusion and incomplete vessel wall apposition compared to angiography alone, thus helping to minimize stent thrombosis.3 OCT helps to confirm that stent is fully expanded to reduce stent failure.1
PCI guidance with OCT is easier now with the development of the standardized step-by-step workflow (also referred to as algorithm), MLD MAX, which is mnemonic for Morphology, Length, Diameter, Medial Dissection, Apposition and Xpansion. Using OCT with MLD MAX workflow can improve stent expansion4 without additional contrast while reducing radiation exposure compared to angiography-guided PCI.5 Stent expansion is linked to better PCI outcomes.6
For references, refer to the original document.
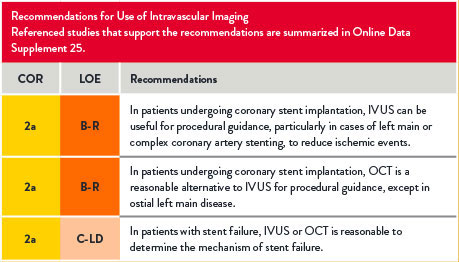
Use of Intravascular Imaging
For references, refer to the original document.
The Basics of OCT
What are the OCT components?
OCT imaging systems consist of three main components: the software, the system, and the catheter.
OCT Software
Ultreon™ Software includes auto detection of certain components of the vessel based on artificial intelligence. The software is intended to be used only with compatible OPTIS™ Next Imaging Systems.
OPTIS™ Next Imaging Systems
The hardware that runs the OCT software. OPTIS™ Systems use optical imaging catheters that emit near-infrared light to produce high-resolution real-time images. The OPTIS™ Next Imaging Systems can be integrated with the cath lab angio systems to display OCT and angio co-registration (ACR) on the same screen.
OCT Catheter
The OCT catheter is intended for the imaging of coronary arteries.

OCT Image Acquisition and Interpretation
To perform an OCT pullback, an OCT catheter is inserted into the vessel and an infrared laser is used to scan the vessel wall in a spiral-like manner. The laser beam penetrates the tissue 2-3mm deep, is reflected from there and returned to the OCT device via the catheter for evaluation.7
How to set up the system for OCT-guided PCI
Watch this video to learn how to set up the OCT system. These step-by-step instructions are available on the OCT screen for easy guidance.
There are four steps to an OCT-guided PCI set up:
- Connecting syringe
- Purging the catheter
- Draping the DOC
- Connecting the catheter
How to initiate a pullback
Watch this video to learn how to initiate a pullback. The step-by-step instructions are also available on the OCT screen.
Due to the OCT systems’ high acquisition speed, images of pullback can be produced and visualized in a matter of seconds. The system provides precise information about the scanned vessel segment.7
How to interpret OCT images
Learn how to interpret OCT images, the basic elements of an OCT image and an algorithm for image interpretation.
OCT and MLD MAX Workflow
Using OCT with MLD MAX workflow, the standardized step-by-step workflow, helps to guide pre- and post-PCI decisions. Use of the workflow resulted in an 88% change in treatment decisions8 compared to angiography alone without a change in contrast usage and a 10% reduction in radiation5, as shown in the LightLab Clinical Initiative.
Six letters represent six steps of the PCI goal to MAX-imize stent expansion to deliver optimal results.
- The first three steps, MLD (Morphology, Length, Diameter) performed before PCI, designed to help inform treatment strategy.
- The last three steps, MAX (Medial Dissection, Apposition, Xpansion), performed post-PCI, to optimize stent placement. MLD MAX was developed as a part of the LightLab Clinical Initiative.
Achieving optimal expansion is proven to reduce rates of major adverse cardiac events during PCI9. Proper expansion confirmed by imaging results in safety and efficacy benefits.6
Morphology
Search for High Calcium1
Criteria:
>180 degrees, and
>0.5 mm thickness, and
>5 mm in length
Length
Select Landing Zones Based on Healthy Tissue/ EEL Visualization2
Place landing zones in healthy tissue (i.e. EEL visualization)
Note: In the absence of EEL to represent healthy tissue find the largest lumen to avoid areas of TCFA or lipid pools so as to not land your stent edge in these high-risk areas3
Diameter
Measure Vessel,
Stent, Balloon Diameters4
Use distal reference measurements to select stent diameter
Use distal reference measurement for distal balloons or proximal reference measurements for proximal balloons
Medial
Dissection
Address Significant Dissection2
Criteria:
Dissection penetrates medial layer, and is greater than 1 quadrant arc
Apposition
Address Gross Malapposition
Criteria:
Malapposition indicator shows longer than 3 mm4 of significant (≥0.3 mm from wall5) apposition
Xpansion
Confirm
Expansion3,6
Criteria:
≥80% acceptable,
≥90% expansion is optimal
“When we use the OCT workflow (MLD MAX), we make better decisions initially by analyzing the plaque morphology and determining appropriate vessel prep as well as selecting the appropriate stent length and diameter. By doing this, we are less likely to have to do post-PCI optimization because we are doing it right the first time. This often leads to improved procedural efficiency.”
— DR. JASON WOLLMUTH, INTERVENTIONAL CARDIOLOGIST
How to Apply MLD MAX Workflow to OCT-Guided PCI?
Watch these videos to learn more about each step of the MLD MAX workflow: Morphology, Length, Diameter, Medial dissection, Apposition, and Xpansion.
Pre-PCI Guidance: MLD | Morphology, Length, Diameter are used to determine PCI strategy.
Morphology
What is the value of morphology-guided lesion prep?
- Understanding vessel morphology and plaque characteristics (lipidic, fibrotic or calcific) allows user to determine vessel preparation strategy (i.e. if direct stenting is okay or if plaque modification is required).
- In this video, Dr. Ziad Ali provides a detailed overview of the structure of the normal vessel morphology, how it displays on OCT and shares an easy-to-use OCT image interpretation algorithm.
- Dr. Ziad Ali discusses vessel preparation strategies based on plaque type, and the influence on coronary calcium on stent expansion. Learn more about OCT in calcified lesions.
Length
Why does proper stent length matter?
Diameter
Why does accurate diameter matter?
- Appropriate sizing of stent diameter is important to limit underexpansion, malapposition and dissections.
- Angiography both over- and underestimates stent diameter to a similar degree and led to an inaccurate stent diameter in 38% of stented lesions as demonstrated in the LightLab Clinical Initiative.8
- In this video, Dr Ziad Ali shows how to measure diameter with an easy-to-use sizing algorithm.
Post-PCI Guidance: MAX | Medial dissection, Apposition, Xpansion are used to optimize stent placement to ensure optimal expansion.
Medial Dissection
There are three types of dissections: intimal, medial and intramural hematoma. A dissection which penetrates the medial layer and > 1 quadrant arc needs to be treated although various recommended angles exist, including > 60 degrees.9 A simplified approach used by operators is to measure a 1 quadrant arc.
Xpansion
An important aspect of optimizing PCI is the detection of underexpansion after stent placement.9
- Underexpansion is a predictor of adverse events (i.e. stent thrombosis and restenosis). Achieving optimal expansion is proven to reduce rates of adverse events.9
- In this video, Dr. Ziad Ali shows how to confirm stent expansion using OCT.
References
*As compared to AptiVue™ Software
- Reyes, M. The next innovation in PCI is not a stent. The value of OCT. CathLab Digest. Oct 6, 2019. Volume 27, Issue 10.
- Ali, Z. et al. Intracoronary optical coherence tomography: state of the art and future directions. EuroIntervention 2021;17:e105-e123. DOI: 10.4244/EIJ-D-21-00089
- AptiVue Software IFU. Refer to Instructions For Use (IFU) for additional information.
- Khuddus, M. et al. Cardiac Catheterization Laboratory Efficiency and Quality Improvement during Percutaneous Coronary Intervention (PCI) Utilizing a Standardized Optimal Coherence Tomography (OCT) Workflow in a Real-World Setting: Results from the LightLab Initiative. CRT 2021.
- Rauch A Standardized Optical Coherence Tomography Workflow Improves Procedural Efficiency and Safety During Percutaneous Coronary Intervention: Insights from the LightLab Clinical Initiative. TCT2021.
- Zhang J, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: the ULTIMATE trial. J Am Coll Cardiol. 2018;72(24):3126-3137.
- Nef, Holger. OCT Compendium. Chapter 1. Systemic OCT image evaluation. First edition, 2016, Germany.
- Bezerra, H. et al: Analysis of changes in decision-making process during OCT-guided PCI -Insights from the LightLab Initiative. EuroPCR2020 Presentation.
- Räber L, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2018;39(35):3281-3300.
- Prati, F. et al. The CLI-OPCI II Study. JACC: Cardiovascular Imaging, 2015: Vol 8, No. 11:1297-305.
References - MLD MAX graphic
- Fujino, A. et al. A new optical coherence tomography-based calcium scoring system to predict stent under expansion. EuroIntervention, April 2018; 13(18):e2182-e2189.
- Prati, F. et al. The CLI-OPCI II Study. JACC: Cardiovascular Imaging, 2015: Vol 8, No. 11:1297-305.
- Kubo, T. et al. Application of Optical Coherence Tomography in Percutaneous Coronary Intervention. Circulation Journal, September 2012: Vol. 76, 2076-2083.
- Ali, Z. et al. ILUMIEN III: Optimize PCI. Lancet 2016, 388:2618-2628.
- Souteyrand, G. et al. PESTO French Registry. European Heart Journal, 2016:37:1208-1216.
- Meneveau, N. et al. DOCTORS Study. Circulation, September 2016, 134:906-917.; Zhang, J. et al. The ULTIMATE Trial. Journal of the American College of Cardiology, Dec 2018: Vol 72, No 24:3126-37.; Russo, R. et al. The AVID Trial. Circ Cardiovasc Intervent, April 2009; 2:113-123.; De Jaegere, P. et al. MUSIC Study. European Heart Journal, February 1998:19,1214-1223.
MAT-2113908 v3.0












