The goal of Abbott’s Investigator Sponsored Study (ISS) program is to enhance clinical and/or scientific knowledge of Abbott products and related disease states to ultimately benefit patients.
Upon request, we may provide support to the institution or employer of the sponsor/investigator conducting the clinical study (pending Abbott review and full approval). Concepts or protocols must be submitted for Abbott's consideration in accordance with company review processes.
For support consideration of pre-clinical research projects, please contact your local Abbott representative for more information or contact customer service to be referred to an Abbott representative.
Submission Process
The diagram below gives a high-level overview of the proposal process.
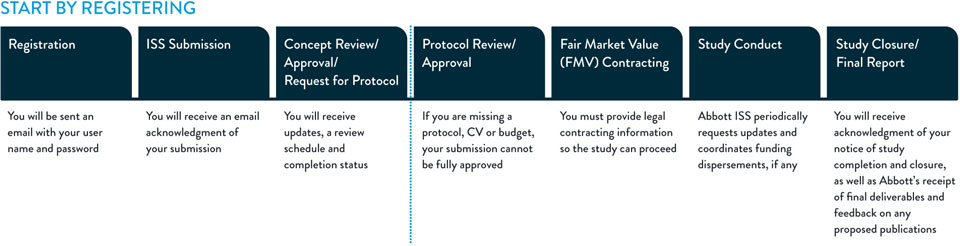
In order for Abbott to review your submission, the submission text and accompanying documents attached must be in the English language.
Application Requirements
The following information is required for application submission:
- Concept or protocol
- Type of support requested from Abbott
- Specification on whether the study is multi-funded
- Curriculum Vitae (CV)
- W-9 form for (US) institution or requestor
- Any relevant past publications
Who May Apply
The following types of organizations are eligible to submit requests for Investigator Sponsored Studies support:
- Medical or professional associations
- Community health centers
- Patient associations or advocacy groups
- Universities or colleges
Provided Support
An applicant can request support for the following:
- Financial support
- Free of cost devices
- Data sharing
Overhead / Indirect Costs
Abbott discourages the imposition of an overhead/indirect charge for studies sponsored and conducted by the institution itself. Any suggested overhead/indirect charge obligation will be examined critically for reasonability in light of Abbott’s proposed funding support.
Inventions
Abbott respects the sponsor’s pre-existing rights in any intellectual property and will not seek to acquire or share any such rights. However, any discoveries or inventions related to an Abbott product used in the study must belong exclusively to Abbott. The sponsor will own the data and the results of the study but must agree to provide Abbott with reports or de-identified protocol-specified information (raw datasets or images) for Abbott’s use in further research or other lawful purposes.
Submit a Concept Document
To submit a concept document/protocol proposal, please follow the instructions provided on the submissions' page.
MAT-1900047 v5.0
