Experience the OCT difference.
Request your Abbott Sales rep today.
OCT-guided PCI Improved Procedural Outcomes and Safety Results as Demonstrated in ILUMIEN IV,1 OCTOBER2
OCT-guided PCI improved stent implantation results with larger stent area and stent expansion1
OCT reduced procedural complications1:
- Major dissections
- Major malapposition
- Major tissue protrusion
- Untreated focal reference segment disease
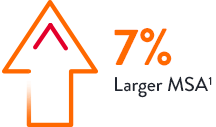
Stent expansion impacts outcomes. Achieving optimal stent expansion (MSA area ≥ 90%) is proven to reduce rates of adverse cardiac events.3 Stent underexpansion is an indicator of adverse events, such as stent thrombosis and restenosis.3
OCT-guided PCI improved safety including reduced stent thrombosis (ST) rates as observed in ILUMIEN IV1,4
- Significant reduction in ST 0.5% vs 1.4%, p=0.02 compared to angiography4

Stent thrombosis is a severe PCI complication that can lead to death or myocardial infarction.5
OCT-guided PCI improved clinical outcomes in complex bifurcation lesions with reduced MACE rates, as demonstrated in OCTOBER2
OCT-guided PCI reduced MACE by 28% compared to angiography2

OCT and IVUS showed comparable clinical results in OCTIVUS6
OCT-guided PCI demonstrated comparable results to IVUS-guided PCI in TVF at 1 year.6
- TVF 2.5% OCT vs 3.1% IVUS, p<0.001 for non-inferiority at 1 year
- Fewer major procedural complications in the OCT group (2.2% vs 3.7%)
- Reduced procedural time in the OCT group
- No differences in contrast-related risk between OCT and IVUS
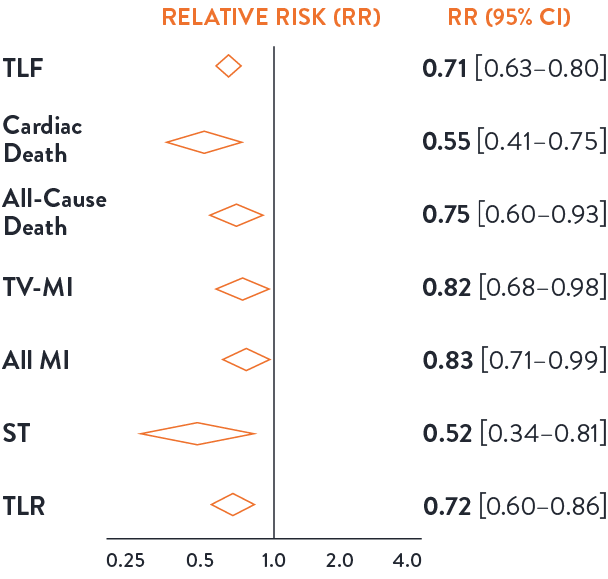
Intravascular imaging to guide PCI is superior to angiography guidance, as demonstrated in the LANCET Network Meta-Analysis7
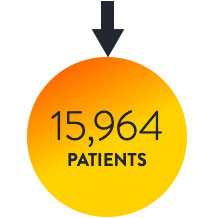
Routine use of intravascular imaging to guide PCI is superior to angiography and has shown to improve patient outcomes consistently in the network meta-analysis from 22 RCTs and 15,964 patients.7
Imaging guidance leads to lower risk of adverse events across efficacy and safety endpoints.7 Imaging guidance has shown a significant reduction in all-cause death (45%) and all-cause MI (25%).
Image-guided PCI leads to lower risk of adverse events across safety & efficacy endpoints compared to angiography7
Reduction:
45% Cardiac death
18% TV-MI
28% TLR

Safety Benefits of Imaging:
48% Reduction ST
25% Reduction all cause mortality
17% Reduction MI

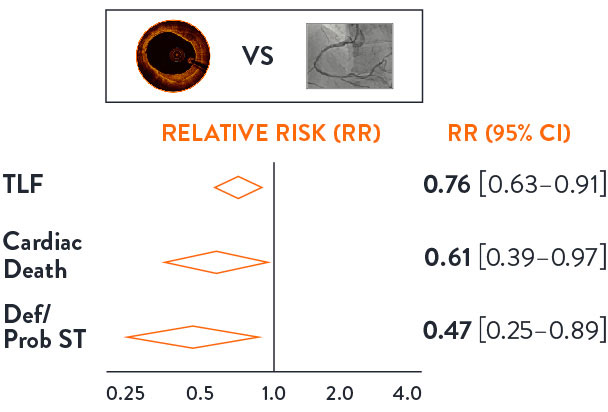
OCT guidance significantly reduces TLF, Cardiac Death and Definite/Probable Stent Thrombosis7
OCT-guided PCI demonstrated 24% reduction in TLF, 39% reduction in Cardiac Death and 53% reduction in definite and probable stent thrombosis, as demonstrated in the LANCET Network Meta-Analysis7
Intravascular Imaging: Can We Still Ignore the Evidence?
Watch Dr. Alasnag review all imaging clinical data presented at the European Society of Cardiology (ESC) 2023.
Imaging matters: large body of data supports imaging benefit
Intravascular imaging data from over 15,000 patients solidifies the notion that intravascular imaging (IVI) should be the gold standard of care for better patient outcomes.
ILUMIEN IV1
OCT-guided PCI vs. angio-guided PCI in complex cases
OCTOBER2
OCT-guided PCI vs. angio-guided PCI in bifurcations
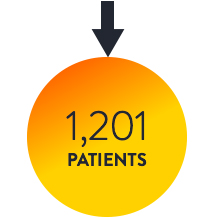
OCTIVUS6
OCT-guided PCI vs. IVUS-guided PCI
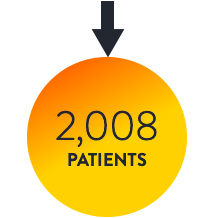
THE LANCET META7
OCT-guided PCI vs. IVUS-guided PCI vs. angio alone
Societies and guidelines recommend imaging guidance for PCI
- 2024 SCAI Expert Consensus Statement on the Management of Calcified Coronary Lesions recommends intravascular imaging in calcified Coronary Artery Disease to assess calcium severity to determine modification strategy, and to understand mechanism of stent failure and calcium distribution in ISR cases.12
- 2023 ESC Guidelines for The Management of Acute Coronary Syndromes recommend intravascular imaging to guide PCI and recommend OCT to guide PCI in patients with ambiguous culprit lesions.8
- 2023 ACC/AHA/SCAI Advanced Training Statement on Interventional Cardiology now require the knowledge of imaging and physiology devices, how to perform imaging-guided PCI as well as the outcomes data to support the use.9
- ACC recommends that operators use IVI as an essential adjunct to angiography, that all US cardiac catheterization labs have IVI capability and national training programs provide training on IVI.10
- The AHA/ACC 2021 Guidelines for use of intravascular imaging were updated and now recommend OCT imaging to guide stent implantation and to determine mechanism of stent underexpansion.11
Clinical Evidence in Detail

References
- Z Ali et al., Optical Coherence Tomography–Guided versus Angiography-Guided PCI, NEJM, DOI: 10.1056/NEJMoa230586
- N.R. Holm et al., OCT or Angiography Guidance for PCI in Complex Bifurcation Lesions, NEJM, DOI: 10.1056/NEJMoa2307770 (OCTOBER)
- Räber L, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2018;39(35):3281-3300.
- Z Ali et al., Optical Coherence Tomography–Guided versus Angiography-Guided PCI. ESC2023 Presentation.
- Souteyrand, G. et al. Mechanisms of stent thrombosis analysed by optical coherence tomography: insights from the national PESTO French registry. European Heart Journal, 2016:37:1208-1216.
- D Kang et al., Optical Coherence Tomography-Guided or Intravascular Ultrasound Guided Percutaneous Coronary Intervention: The OCTIVUS Randomized Clinical Trial, 10.1161/CIRCULATIONAHA.123.066429
- Stone, et al., Intravascular Imaging-guided coronary drug-eluting stent implantation: an updated network meta-analysis, The Lancet, doi.org/10.1016/ S0140-6736(23)02454-6
- 2023 ESC Guidelines for the management of acute coronary syndromes. Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). European Heart Journal (2023) 00, 1–107. doi.org/10.1093/eurheartj/ehad191
- 2023 ACC/AHA/SCAI Advanced Training Statement on Interventional Cardiology (Coronary, Peripheral Vascular, and Structural Heart Interventions): A Report of the ACC Competency Management Committee | Circulation: Cardiovascular Interventions. 2023;0:e000088 https://doi.org/10.1161/HCV.0000000000000088.
- Truesdell AG, Alasnag MA, Kaul P, et al. Intravascular Imaging During Percutaneous Coronary Intervention: JACC State-of-the-Art Review. J Am Coll Cardiol. 2023;81(6):590-605. doi:10.1016/j.jacc.2022.11.045.
- 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e18–e114. DOI: 10.1161/CIR.0000000000001038.
- Riley, R, et al. SCAI Expert Consensus Statement on the Management of Calcified Coronary Lesions. JSCAI, 2024. https://doi.org/10.1016/j.jscai.2023.101259
MAT-2311278 v3.0
Important Safety Information
OPTIS™ and OPTIS™ Next Imaging Systems and Software

INDICATIONS
Applies to OPTIS™ Imaging Systems and Software
The OPTIS™ Software and AptiVue™ E Series Software are intended to be used only with compatible OPTIS™ Imaging Systems.
The OPTIS™ Imaging Systems with a compatible Dragonfly™ Imaging Catheter are intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The compatible Dragonfly™ Imaging Catheters are intended for use in vessels 2.0 to 3.5 mm in diameter. The compatible Dragonfly™ Imaging Catheters are not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
The OPTIS™ Imaging Systems are intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise and clinical judgment to determine if therapeutic intervention is indicated.
Applies to OPTIS™ Next Imaging Systems and Software
The Ultreon™ 1.0 Software and Ultreon™ 2.0 Software are intended to be used only with compatible OPTIS™ Next Imaging Systems.
The OPTIS™ Next Imaging System with a compatible Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
The OPTIS™ Next Imaging Systems are intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise, and clinical judgment to determine if therapeutic intervention is indicated.
Applies to both OPTIS™ and OPTIS™ Next Imaging Systems and Software
The Dragonfly™ OPTIS™ or Dragonfly™ OpStar™ Imaging Catheters are intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly™ OPTIS™ or Dragonfly™ OpStar™ Imaging Catheters are not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
The OPTIS™ and OPTIS™ Next Imaging Systems are intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise, and clinical judgment to determine if therapeutic intervention is indicated.
CONTRAINDICATIONS
The OPTIS™ and OPTIS™ Next Integrated Systems and Mobile Systems with the usage of the OPTIS™ Software, AptiVue™ E Series Software, Ultreon™ 1.0 Software, and Ultreon™ 2.0 Software are contraindicated where introduction of any catheter would constitute a threat to patient safety. Contraindications include:
- Bacteremia or sepsis
- Major coagulation system abnormalities
- Patients diagnosed with coronary artery spasm
- Patients disqualified for coronary artery bypass graft (CABG) surgery
- Patients disqualified for percutaneous transluminal coronary angioplasty (PTCA)
- Severe hemodynamic instability or shock
- Total occlusion
- Large thrombus
- Acute renal failure
- Inability to tolerate systemic anticoagulation is a contraindication to use of OCT for coronary imaging.
- The system has no patient alarm functions. Do not use for cardiac monitoring.
COMPLICATIONS
The following complications may occur as a consequence of intravascular imaging and catheterization procedure:
- Abnormal heart rhythm or arrhythmias
- Acute myocardial infarction
- Allergic reaction to the contrast media or drug administered for the procedure
- Arterial dissection, injury, or perforation
- Bleeding
- Catheter access site reactions: inflammation or granuloma
- Coronary artery spasm
- Death
- Embolism
- Hypotension
- Infection
- Myocardial ischemia
- Renal insufficiency or failure from contrast media use
- Repeat revascularization
- Thrombus formation, abrupt closure, or total occlusion
- Tissue necrosis
- Unstable angina
WARNINGS
- Prior to use, please review the Instructions for Use supplied with the OPTIS™ imaging system, Dragonfly™ Imaging Catheter, Wi-Box™ AO Transmitter and the PressureWire™ guidewire for more information.
- The Dragonfly™ Imaging Catheter is sterilized by ethylene oxide and is intended for one time use only. Non-pyrogenic. Do not use if the package is opened or damaged. Do not reuse or re-sterilize. Any attempt to reuse or re-sterilize may compromise the structural integrity of this device. Adverse effects of using a non-sterile or re-sterilized catheter may include, but are not limited to: local and / or systemic infection, mechanical damage, inaccurate results.
- Appropriate anticoagulant and vasodilator therapy must be used during the procedure as needed.
- Ensure that no air is introduced into the system during the Dragonfly™ Imaging Catheters insertion.
- Observe all advancement and movement of the Dragonfly™ Imaging Catheters under fluoroscopy. Always advance and withdraw the catheter slowly. Failure to observe device movement fluoroscopically may result in vessel injury or device damage. To ensure proper placement, do not move the guide wire after a Dragonfly™ Imaging Catheter is in place.
- If resistance is encountered during advancement or withdrawal of the Dragonfly™ Imaging Catheter, stop manipulation and evaluate under fluoroscopy. If the cause of resistance cannot be determined or mitigated, carefully remove the Dragonfly™ Imaging Catheters and guidewire together as a unit from the patient.
- Leave the guide wire engaged with a Dragonfly™ Imaging Catheter at all times during use. Do not withdraw or advance the guide wire prior to withdrawing the Dragonfly™ Imaging Catheters.
- The Dragonfly™ Imaging Catheters should never be forced into lumens that are narrower than the Dragonfly™ Imaging Catheters body or forced through a tight or heavily calcified lesion.
- The Dragonfly™ Imaging Catheters should not be advanced through abnormally tortuous anatomy.
- When advancing or retracting a Dragonfly™ Imaging Catheter with a monorail tip through a stented vessel, the Dragonfly™ Imaging Catheters may engage the stent between the junction of the Dragonfly™ Imaging Catheters and guide wire, resulting in entrapment of catheter / guide wire, catheter tip separation, stent dislocation, and / or vascular injury.
- Refer to the contrast media Instructions for Use for general warnings and precautions relating to use of contrast media.
- Before creating an OCT recording, review “Performing an OCT Procedure” for additional warnings and cautions in the IFU.
PRECAUTIONS
- Safety and effectiveness have been established for the following patient population: adult patients undergoing non-emergent percutaneous coronary interventions in lesions with reference vessel diameters between 2.0 to 3.5 mm, which are not located in the left main coronary artery or in a target vessel which has undergone previous bypass procedures.
- Follow all instructions, warnings, and cautions provided in “Patient Safety” in the IFU.
- All operators must be knowledgeable in performing OCT and physiological procedures prior to using the OPTIS™ and OPTIS™ Next Integrated Systems and Mobile Systems with the usage of the OPTIS™ Software, AptiVue™ E Series Software, Ultreon™ 1.0 Software, and Ultreon™ 2.0 Software.
- When using saline, heparinized saline is recommended.
- Monitor the OCT image for indications of the Dragonfly™ Imaging Catheters optical failure. If optical failure is suspected, remove the Dragonfly™ Imaging Catheter from the patient, press “Unload” on the drive motor and optical controller (DOC), detach the catheter, and replace it with a new one.
- If the pullback triggers before contrast is injected, repeat the pullback.
- For optimal imaging, only use 100% contrast media.
- Use the minimum flush rate and volume required to image the desired anatomy.
- To obtain accurate measurements, be sure the selection for the Flush Medium is the same as the medium in which you are imaging.
- The Dragonfly™ Imaging Catheters must be purged prior to connection to the DOC to prevent damage to the imaging core.
- Do not insert or remove a Dragonfly™ Imaging Catheter while the DOC is scanning. Do not attempt to disconnect the catheter from the DOC while the “lock” LED is blinking as it could damage the catheter or the DOC. Refer to “Removing the Dragonfly™ Imaging Catheter” in the IFU.
- Never attempt to attach or detach a catheter to the DOC while the "lock" LED is lit.
- Take care in handling the Dragonfly™ Imaging Catheters to prevent breaking the fiber-optics within the catheter. Kinking and bending of the catheter can cause damage. While connecting, ensure the proximal catheter segment is straight and aligned with the DOC. Never attempt to connect and operate the catheter while the catheter remains coiled within the hoop.
- Do not kink, sharply bend, pinch, or crush the Dragonfly™ Imaging Catheters at any time.
- The Dragonfly™ Imaging Catheters have no user serviceable parts. Do not attempt to repair or alter any part of the catheter assembly as provided.
- If you want to make measurements on files that will be exported to standard formats, you must make the measurements BEFORE exporting the images. Using non-OCT software to measure standard format images will not produce accurate measurements.
- Do not use images that have been exported to JPEG or Compressed AVI formats for clinical decision making. These formats use compression methods that may degrade the image quality.
- Artifacts may result in misrepresentation of L-mode data, so L-mode is not recommended for quantification of clinical information.
- It is the user’s responsibility to confirm the lumen contours of all the frames within the reference segment, and to make adjustments if necessary. Red frames indicate low confidence in the detected contours.
- Deleted files cannot be restored. After files have been deleted, they can only be imported back to your system from your archived copies.
- Restoring factory default settings resets ALL user-entered configuration values except the date and time. This button should be used only under the direction of qualified service personnel.
MAT-2309288 v1.0
