Experience the OCT difference.
Request your Abbott Sales rep today.
OCT Education Library
Explore additional educational resources to learn more about OCT imaging in a variety of formats.
Watch:
Social Media and OCT-ober
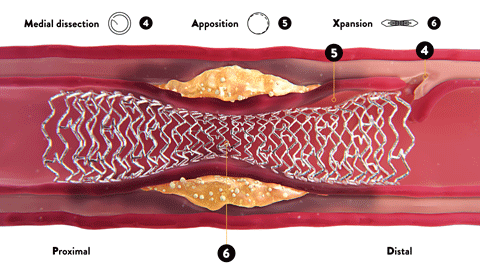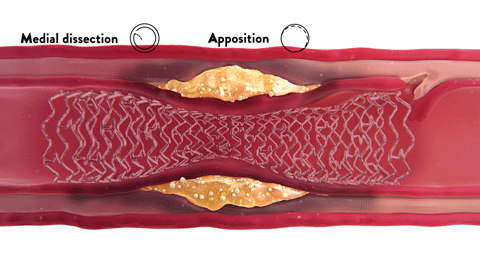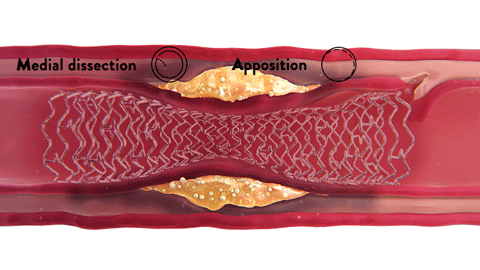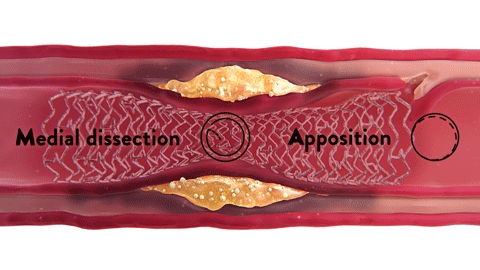
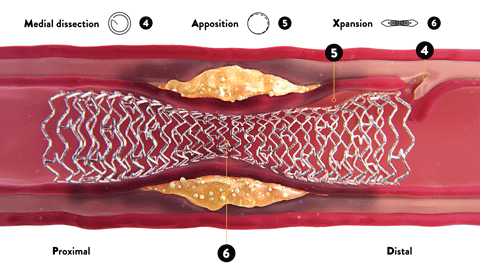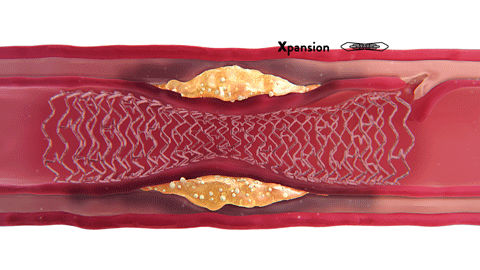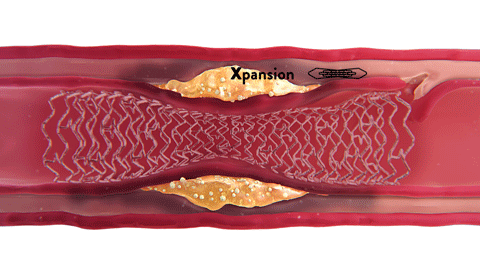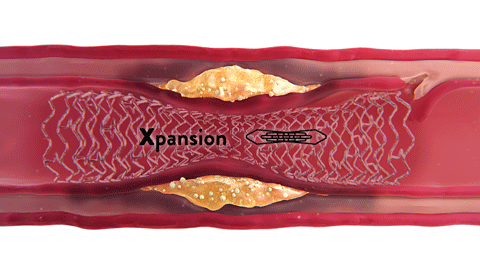
OCT-ober is Abbott’s annual educational initiative on Twitter ![]() @AbbottCardio to teach about OCT and highlight the role and impact of image-guided PCI. Launched in 2018, the campaign has established #OCTimaging hashtag for OCT-related conversations. Since then, it has been used in more than 2K tweets and reached over 4MM impressions,* amplifying the awareness of imaging across the globe.
@AbbottCardio to teach about OCT and highlight the role and impact of image-guided PCI. Launched in 2018, the campaign has established #OCTimaging hashtag for OCT-related conversations. Since then, it has been used in more than 2K tweets and reached over 4MM impressions,* amplifying the awareness of imaging across the globe.
The content is published in a form of short tweetorials (a thread of tweets) and covers a range of topics such as the basics of OCT, why optimize PCI with OCT, how to diagnose coronary calcium with OCT, how to identify in-stent restenosis (ISR) with OCT and more.
Check out these short, digestible threads by year and topic:
2018: The Basics of OCT Imaging
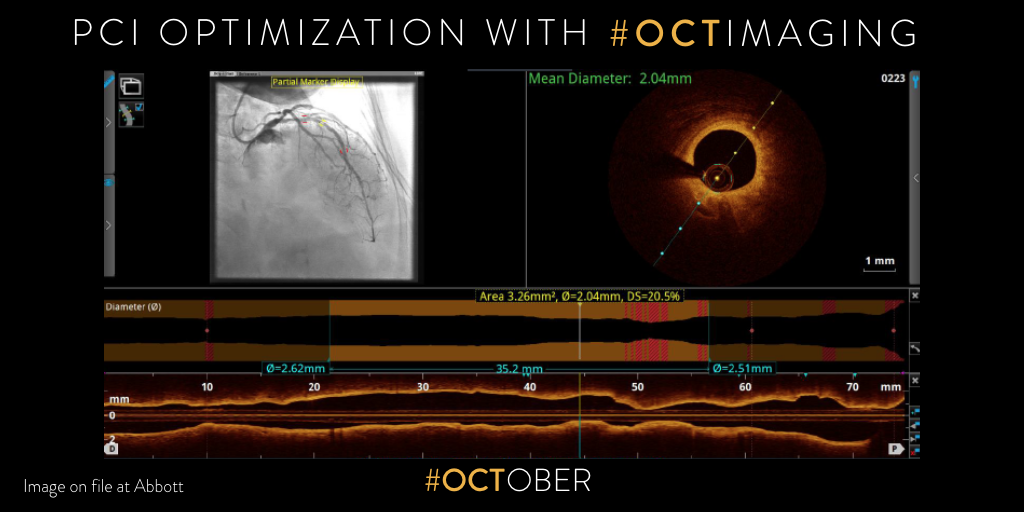
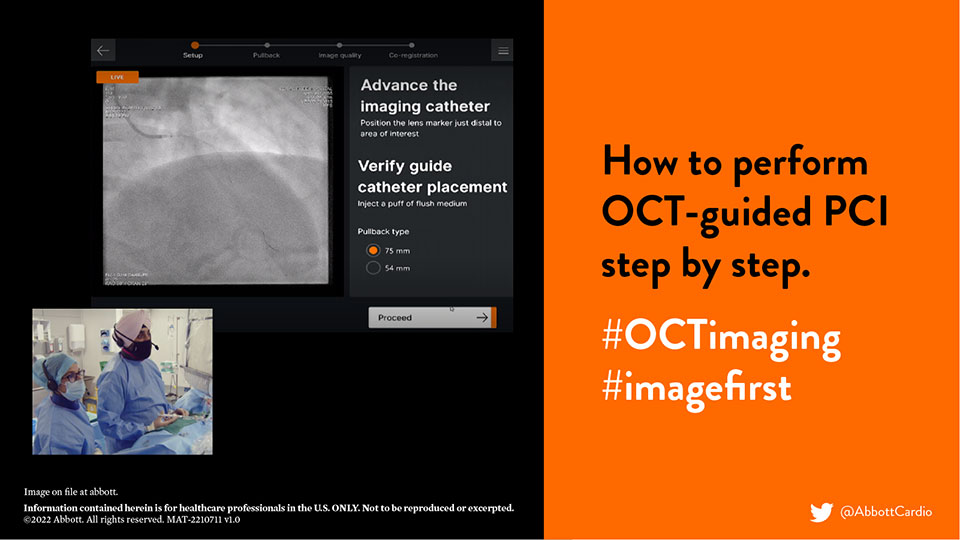
2019: Why and How to optimize PCI with #OCTimaging
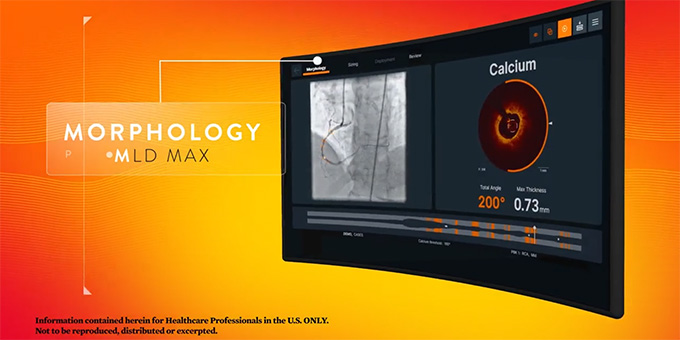
2020: OCT and MLD MAX Workflow
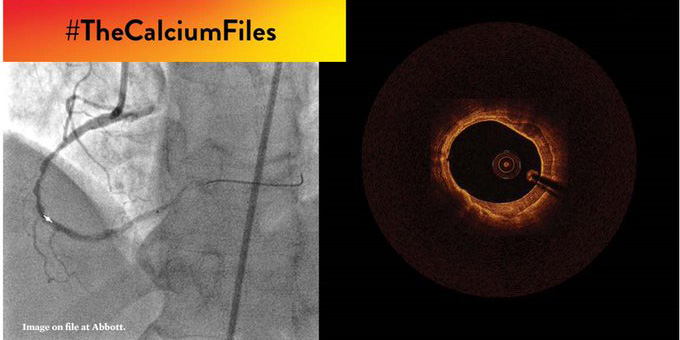
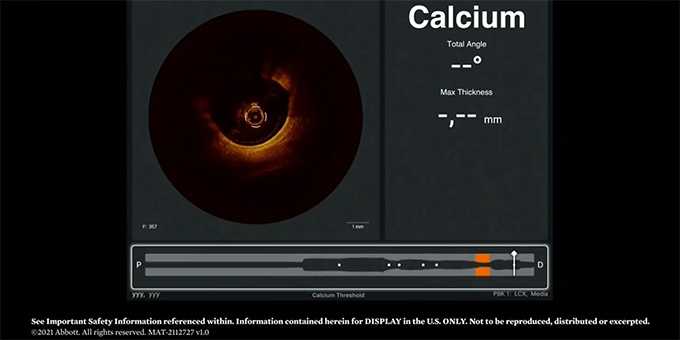
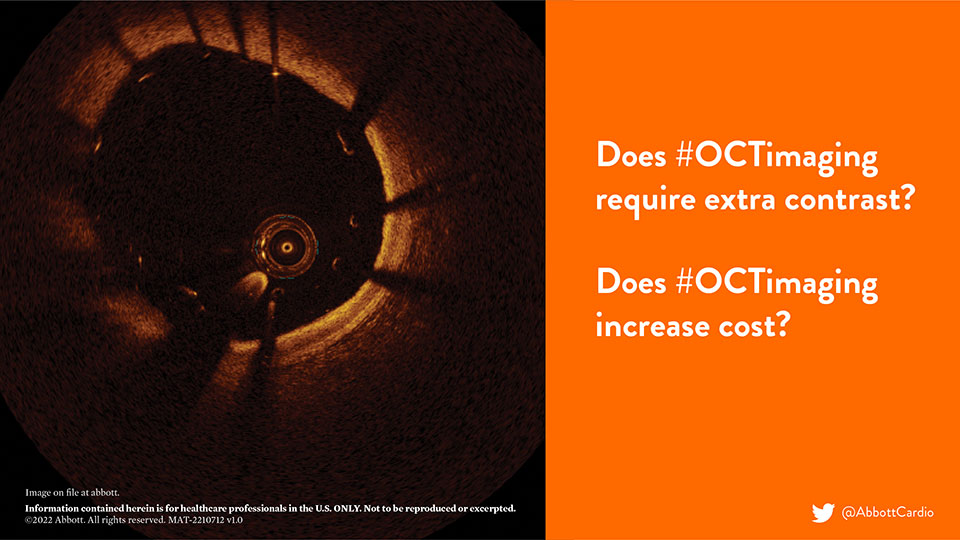
2021: OCT and Coronary Calcium #TheCalciumFiles
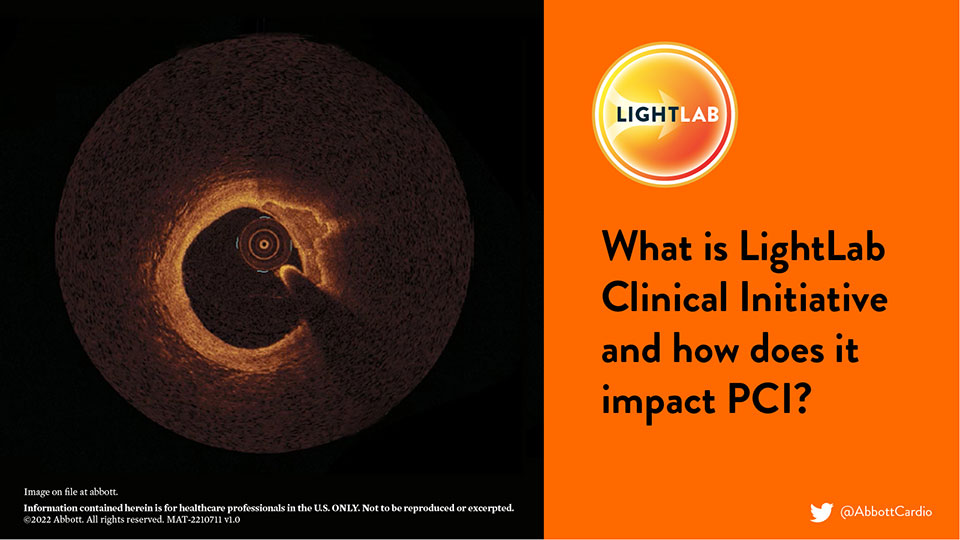
2022: OCT-guided PCI, LightLab and MLD MAX Workflow
References:
*Symplur Data. #OCTimaging Hashtag. 2018-2022.
MAT-2113913 v3.0
Dragonfly™ OPTIS™ Imaging Catheter

Indications: The Dragonfly™ OPTIS™ Imaging Catheter with the OCT Imaging System is intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The Dragonfly™ OPTIS™ Imaging Catheter is intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly™ OPTIS™ Imaging Catheter is not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
Contraindications: Use of the Dragonfly™ OPTIS™ Imaging Catheter is contraindicated where introduction of any catheter would constitute a threat to patient safety. Contraindications include:
- Bacteremia or sepsis
- Major coagulation system abnormalities
- Patients disqualified for CABG surgery
- Patients disqualified for PTCA
- Severe hemodynamic instability or shock
- Patients diagnosed with coronary artery spasm
- Total occlusion
- Large thrombus
- Acute renal failure
Warnings and Precautions:
- Store at ambient temperature in a dry location out of direct sunlight.
- Ensure the catheter is at room temperature (10°C to 32°C) before use.
- This device is sterilized by ethylene oxide and is intended for one time use only. Non-pyrogenic. Do not use if the package is opened or damaged. Do not reuse or re-sterilize.
- Appropriate anticoagulant and vasodilator therapy must be used during the procedure as needed.
- Observe all advancement and movement of the Dragonfly™ OPTIS™ Imaging Catheter under fluoroscopy. Always advance and withdraw the catheter slowly. Failure to observe device movement fluoroscopically may result in vessel injury or device damage. To assure proper placement do not move the guide wire after the Dragonfly™ OPTIS™ Imaging Catheter is in place.
- If resistance is encountered during advancement or withdrawal of the Dragonfly™ OPTIS™ Imaging Catheter, stop manipulation and evaluate under fluoroscopy. If the cause of resistance cannot be determined or mitigated, carefully remove the catheter from the patient.
- Use the minimum flush rate and volume required to image the desired anatomy.
- After use, this product may be a potential biohazard. Handle and dispose of in accordance with accepted medical practice and applicable laws and regulations.
- The instructions for use are recyclable. Dispose of all packaging materials appropriately.
- Refer to contrast media Instructions for Use for general warnings and precautions relating to contrast media.
- This package contains a Sterile Single Use Only Device. Any attempt to re-use or re-sterilize may compromise the structural integrity of this device.
- Do not remove the Dragonfly™ OPTIS™ Imaging Catheter from the DOC until the procedure is complete to avoid a potential sterility breach.
- Always verify that the catheter has been properly prepared prior to inserting into vasculature.
Potential Adverse Events: The following complications may occur as a consequence of intravascular imaging:
- Coronary artery spasm
- Unstable angina
- Allergic reaction to the contrast media
- Arterial dissection, injury or perforation
- Thrombus formation, abrupt closure, or total occlusion
- Abnormal heart arrhythmias
- Embolism
- Acute myocardial infarction
- Death
MAT-2115908 v2.0
OPTIS™ Imaging Systems and Software

Indications The OPTIS™ Software and AptiVue™ E Series Software are intended to be used only with compatible OPTIS™ Imaging Systems.
The OPTIS™ Imaging System with a compatible Dragonfly™ Imaging Catheter is intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The compatible Dragonfly™ Imaging Catheters are intended for use in vessels 2.0 to 3.5 mm in diameter. The compatible Dragonfly™ Imaging Catheters are not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
The OPTIS™ Imaging System is intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise and clinical judgment to determine if therapeutic intervention is indicated.
Contraindications: The OPTIS™ Integrated System and Mobile System with Software are contraindicated where introduction of any catheter would constitute a threat to patient safety. Contraindications include:
- Bacteremia or sepsis
- Major coagulation system abnormalities
- Patients diagnosed with coronary artery spasm
- Patients disqualified for CABG surgery
- Patients disqualified for PTCA
- Severe hemodynamic instability or shock
- Total occlusion
- Large thrombus
- Acute renal failure
NOTE: The systems have no patient alarm functions. Do not use for cardiac monitoring.
Warnings:
- Appropriate anticoagulant and vasodilator therapy must be used during the procedure as needed.
- Observe all advancement and movement of the Dragonfly™ Imaging Catheter under fluoroscopy. Always advance and withdraw the catheter slowly. Failure to observe device movement fluoroscopically may result in vessel injury or device damage.
- Leave the guidewire engaged with the catheter at all times during use. Do not withdraw or advance the guidewire prior to withdrawing the catheter.
- If resistance is encountered during advancement or withdrawal of the Dragonfly™ Imaging Catheter, stop manipulation and evaluate under fluoroscopy. If the cause of resistance cannot be determined or mitigated, carefully remove the catheter and guidewire together.
- The Dragonfly™ Imaging Catheter should never be forced into lumens that are narrower than the catheter body or forced through a tight or heavily calcified lesion.
- The Dragonfly™ Imaging Catheter should not be advanced through abnormally tortuous anatomy.
- When advancing or retracting a catheter with a monorail tip through a stented vessel, the catheter may engage the stent between the junction of the Dragonfly™ Imaging Catheter and guidewire, resulting in entrapment of catheter/guidewire, catheter tip separation, and/or stent dislocation.
- Refer to the contrast media’s instructions-for-use for general warnings and precautions relating to use of the contrast media.
- To protect the privacy and security of sensitive information, including electronic protected health information (EPHI), and to protect the integrity of the system itself, the system should be located in a physically secure, access controlled environment.
- Do not use the OPTIS™ Imaging System if there is reason to believe the system's security has been compromised or if the system was unaccounted for a period of time (i.e. misappropriated, modified or tampered with).
Precautions:
- Safety and effectiveness have been established for the following patient population: adult patients undergoing non-emergent percutaneous coronary interventions in lesions with reference vessel diameters between 2.0 to 3.5 mm, which were not located in the left main coronary artery or in a target vessel which has undergone previous bypass procedures.
- For optimal imaging, only use 100% contrast media.
- Store the Dragonfly™ Imaging Catheter at ambient temperature in a dry location out of direct sunlight.
- Never attempt to attach or detach a Dragonfly™ Imaging Catheter to the DOC while the “lock” LED is lit.
- Do not kink, sharply bend, pinch, or crush the Dragonfly™ Imaging Catheter at any time.
- The Dragonfly™ Imaging Catheter is for single use only. Do not reuse, re-sterilize, or reprocess.
- The Dragonfly™ Imaging Catheter is sterilized by ethylene oxide and is intended for one time use only. Non-pyrogenic. Do not use if the package is opened or damaged.
- After use, the Dragonfly™ Imaging Catheter may be a potential biohazard. Handle and dispose of in accordance with accepted medical practice and applicable laws and regulations.
- The Dragonfly™ Imaging Catheter has no user serviceable parts. Do not attempt to repair or alter any part of the catheter assembly as provided.
MAT-2115909 v2.0
Dragonfly OpStar™ Imaging Catheter

Indications: The Dragonfly OpStar™ Imaging Catheter with the OCT Imaging System is intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The Dragonfly OpStar™ Imaging Catheter is intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly OpStar™ Imaging Catheter is not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
Contraindications: Use of the Dragonfly OpStar™ Imaging Catheter is contraindicated where introduction of any catheter would constitute a threat to patient safety. Contraindications include:
- Acute renal failure
- Bacteremia or sepsis
- Large thrombus
- Major coagulation system abnormalities
- Patients diagnosed with coronary artery spasm
- Patients disqualified for coronary artery bypass graft (CABG) surgery
- Patients disqualified for percutaneous transluminal coronary angioplasty (PTCA)
- Severe hemodynamic instability or shock
- Total occlusion
- Inability to tolerate systemic anticoagulation is a contraindication to use of OCT for coronary imaging
Warnings:
- Appropriate anticoagulant and vasodilator therapy is recommended to be used during the procedure as ordered by the physician.
- The Dragonfly OpStar™ Imaging Catheter is sterilized by ethylene oxide and is intended for one time use only. Nonpyrogenic. Do not use if the package is opened or damaged. Do not reuse or re-sterilize. Any attempt to reuse or re-sterilize may compromise the structural integrity of this device. Adverse effects of using a non-sterile or re-sterilized imaging catheter may include, but are not limited to:
- Local and/or systemic infection
- Mechanical damage
- Inaccurate results
- Note the product "Use by" date on the package.
- Observe all advancement and movement of the Dragonfly OpStar™ Imaging Catheter under fluoroscopy. Always advance and withdraw the catheter slowly and ensure that the guide wire is coaxial to the monorail. Failure to observe device movement fluoroscopically may result in vessel injury or device damage. To ensure proper placement, do not move the guide wire after the Dragonfly OpStar™ Imaging Catheter is in place.
- If resistance is encountered during withdrawal of the Dragonfly OpStar™ Imaging Catheter:
- Stop manipulation and evaluate under fluoroscopy.
- If guide wire prolapse is observed, readvance the catheter, ensure the guide wire is coaxial - to the monorail, and reattempt withdrawal.
- If the cause of resistance cannot be determined or mitigated, carefully remove the Dragonfly OpStar™ Imaging Catheter and guide wire as a unit from the patient and replace the Dragonfly OpStar™ Imaging Catheter and guide wire. Do not reuse the Dragonfly OpStar™ Imaging Catheter and guide wire.
- Leave the guide wire engaged with the Dragonfly OpStar™ Imaging Catheter at all times during use. Do not retract or advance the guide wire prior to withdrawing the Dragonfly OpStar™ Imaging Catheter.
- The Dragonfly OpStar™ Imaging Catheter should never be forced into lumens that are narrower than the catheter body or forced through a tight or heavily calcified lesion.
- The Dragonfly OpStar™ Imaging Catheter should not be advanced through abnormally tortuous anatomy.
- When advancing or retracting a Dragonfly OpStar™ Imaging Catheter with a monorail tip through a stented vessel, the Dragonfly imaging catheter may engage the stent between the junction of the Dragonfly OpStar™ Imaging Catheter and guide wire, resulting in entrapment of the catheter/guide wire, catheter tip separation, stent dislocation and/or vascular injury.
- Do not remove the Dragonfly OpStar™ Imaging Catheter from the DOC until the procedure is complete to avoid a potential sterility breach.
- Always verify that the Dragonfly OpStar™ Imaging Catheter has been properly prepared prior to inserting into vasculature.
- The safety and effectiveness of the coated device has not been established, or is unknown, in vascular regions other than those specifically indicated.
- Failure to abide by the warnings in this Instructions for Use might result in damage to the device coating, which may necessitate intervention or result in serious adverse events.
Precautions:
- Safety and effectiveness have been established for the following patient population: adult patients undergoing non-emergent percutaneous coronary interventions in lesions with reference vessel diameters between 2.0 mm to 3.5 mm, which were not located in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
- Use the minimum flush rate and volume required to image the desired anatomy.
- For optimal imaging, only use 100% contrast media.
- Refer to contrast media Instructions for Use for general warnings and precautions relating to contrast media.
- Do not kink, sharply bend, pinch, or crush the Dragonfly OpStar™ Imaging Catheter at any time.
- The Dragonfly OpStar™ Imaging Catheter has no user serviceable parts. Do not attempt to repair or alter any part of the Dragonfly OpStar™ Imaging Catheter assembly as provided.
- After use, the Dragonfly OpStar™ Imaging Catheter may be a potential biohazard. Handle and dispose of in accordance with accepted medical practice and applicable laws and regulations.
- When using saline, heparinized saline is recommended. When wet, the hydrophilic coating increases the lubricity of the coated surface.
- Avoid abrasion of the hydrophilic coating. Use caution when manipulating, advancing and / or withdrawing these devices through needles, metal cannulas, stents, or other devices with sharp edges, or through tortuous or calcified blood vessels. Manipulation, advancement, and / or withdrawal past sharp or beveled edges may result in destruction and / or separation of the outer coating, which may lead to clinical adverse events, resulting in coating material remaining in the vasculature or device damage. This may result in adverse events requiring additional intervention.
- The integrity and performance of the device coating can be negatively impacted by preparation with incompatible media or solvents. Please take note of the following important recommendations:
- Avoid wiping the device with dry gauze as this may damage the device coating.
- Avoid excessive wiping of the coated device.
- Avoid using alcohol, antiseptic solutions, or other solvents to pre-treat the device because this may cause unpredictable changes in the coating which could negatively affect the safety and performance of the catheter.
- Do not soak the device as it may adversely impact the hydrophilic coating on the catheter.
- The Dragonfly OpStar™ Imaging Catheter must be purged prior to connection to the DOC to prevent damage to the imaging core.
- Ensure that the Dragonfly OpStar™ Imaging Catheter tip marker has been properly identified and differentiated from the lens marker before contrast administration and prior to performing the OCT reading.
- Never attempt to attach or detach the Dragonfly OpStar™ Imaging Catheter to the DOC while the "lock" LED is lit.
Complications:
The following complications may occur as a consequence of intravascular imaging and catheterization procedure:
- Allergic reaction to the contrast media or drug administered for the procedure
- Bleeding
- Arterial dissection, injury or perforation
- Abnormal heart rhythm or arrhythmias
- Unstable angina
- Coronary artery spasm
- Thrombus formation, abrupt closure, or total occlusion
- Embolism
- Infection
- Myocardial ischemia
- Acute myocardial infarction
- Repeat revascularization
- Renal insufficiency or failure from contrast media use
- Death
- Catheter access side reactions: inflammation or granuloma or tissue necrosis
- Hypotension
MAT-2115499 v3.0
OPTIS™ Next Imaging Systems and Software

Indications
The Ultreon™ 1.0 Software is intended to be used only with compatible OPTIS™ Next Imaging Systems. The OPTIS™ Next Imaging System with a compatible Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure. The OPTIS™ Next Imaging System is intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise, and clinical judgment to determine if therapeutic intervention is indicated.
Contraindications: Use of the Ultreon™ 1.0 Software is contraindicated where introduction of any catheter would constitute a threat to patient safety.
Contraindications include:
- Bacteremia or sepsis
- Major coagulation system abnormalities
- Patients diagnosed with coronary artery spasm
- Patients disqualified for coronary artery bypass graft (CABG) surgery
- Patients disqualified for percutaneous transluminal coronary angioplasty (PTCA)
- Severe hemodynamic instability or shock
- Total occlusion
- Large thrombus
- Acute renal failure
- Inability to tolerate systemic anticoagulation is a contraindication to use of OCT for coronary imaging.
- PressureWire™ Guidewire is contraindicated for use in the cerebral vasculature.
- The system has no patient alarm functions. Do not use for cardiac monitoring.
Complications: The risks involved in vascular imaging include those associated with all catheterization procedures. The following complications may occur as a consequence of intravascular imaging and may necessitate additional medical treatment including surgical intervention.
- Abnormal heart rhythm or arrhythmias
- Acute myocardial infarction
- Allergic reaction to the contrast media or drug administered for the procedure
- Arterial dissection, injury, or perforation
- Bleeding
- Catheter access site reactions: sterile inflammation or granuloma
- Coronary artery spasm
- Death
- Embolism
- Myocardial ischemia
- Renal insufficiency or failure from contrast media use
- Repeat revascularization
- Thrombus formation, abrupt closure, or total occlusion
- Tissue necrosis
- Unstable angina
- Hypotension
Warnings:
- Refer to the contrast media Instructions for Use for general warnings and precautions relating to use of contrast media.
- The heart rate and mean pressure values shown on the OPTIS™ Next Imaging System are for reference only and are not intended to be used as the primary display.
- The system may place the point of index value at the wrong location due to pressure artifacts, for example: abnormal heartbeats, artifacts in AO (Pa) caused by flushing of guiding catheter, or valve opening / closing. The physician should always confirm that the point selected by the system is a valid point for the calculation of index value.
- Inside the catheterization laboratory, only port-powered USB drives may be connected to the USB port. Connecting externally powered devices to the USB port in the patient vicinity may compromise electrical isolation and cause patient injury.
- To protect the privacy and security of sensitive information, including electronic protected health information (EPHI), and to protect the integrity of the system itself, the system should be located in a physically secure, access-controlled environment. Do not use the OPTIS™ Next Imaging System if there is reason to believe the system’s security has been compromised or if the system was unaccounted for during a period of time (i.e., misappropriated, modified, or tampered with).
Precautions:
- Safety and effectiveness have been established for the following patient population: adult patients undergoing non-emergent percutaneous coronary interventions in lesions with reference vessel diameters between 2.0 to 3.5 mm, which were not located in the left main coronary artery or in a target vessel which has undergone previous bypass procedures.
- Monitor the OCT image for indications of Dragonfly™ Imaging Catheter optical failure. If optical failure is suspected, remove the Dragonfly™ Imaging Catheter from the patient, press “Unload” on the drive motor and optical controller (DOC), detach the catheter, and replace it with a new one.
- If the pullback triggers before contrast is injected, repeat the pullback.
- For optimal imaging, only use 100% contrast media.
MAT-2104193 v3.0


Stay Connected