Peripheral Artery Disease (PAD) is a significant global health burden, affecting over 236 million people worldwide1 and presents in 15-20% of people > 70 years of age.2 As a global leader in peripheral intervention, Abbott offers innovative products to treat the full spectrum of PAD and chronic limb threatening ischemia (CLTI).
Every lower limb case is unique, and no two lesions are the same. That is why we focus on providing the therapies, tools, and specialized knowledge that enable you to tailor your approach and optimize long-term, durable outcomes.

Individualized Solutions in Lower Limbs
Our comprehensive portfolio offers individualized solutions for every step in lower limb care from ACCESS to CLOSE.
Access
Gain access with the right guide wires.
Workhorse
- Hi-Torque Command™ 14 Guide Wire Family (.014")
- Hi-Torque Command™ 18 Guide Wire Family (.018")
- Hi-Torque Versacore™ Guide Wire Family (.035")
Specialty
- Hi-Torque Command™ 14 ST Guide Wires (.014")
- Hi-Torque Winn™ 200T Guide Wires (.014")
- Hi-Torque Connect™ 250T Guide Wires (.018")
Supportive
Prepare
Clear obstacles with peripheral dilatation catheters, embolic protection, and orbital atherectomy.
Simple to Complex Lesions
- Armada™ Balloon Dilatation Catheters
- JADE‡ PTA Balloon Catheters
- Emboshield NAV6™ Embolic Protection System
- Diamondback 360™ Peripheral Orbital Atherectomy System
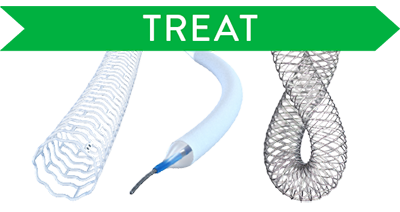
Treat
Treat confidently with DRS, DCB, and nitinol stents.
Treatment Options for Complex Lesions
- Esprit™ BTK Everolimus Eluting Resorbable Scaffold System
- SurVeil‡ Drug-Coated Balloon Catheter
- Supera™ Peripheral Stent System
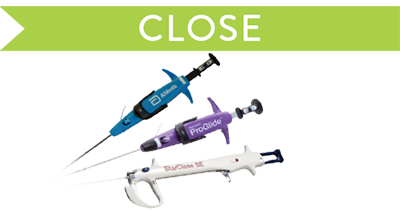
Close
Manage hemostasis efficiently.
Suture-based
- Perclose™ ProStyle™ Suture-Mediated Closure and Repair System
- Perclose ProGlide™ Suture-Mediated Closure System
Clip-based
Additional Abbott Peripheral Products
Sharing Information, Promoting Understanding:
Resources to Help Your Patients Walk Away from PAD/CLTI
Beyond the treatment, make sure your referring physicians are fully aware of PAD and CLTI and how they can help with early diagnosis and timely treatment. For information specifically for the physician, visit our Clear Program webpage. Access a full range of helpful tools and insights. And for your patients, refer them to the PAD-info.com link to support the work you do, so they can learn about PAD symptoms and access an educational brochure.
Clear Program
Physicians' information on how to help their patients understand PAD

About PAD
Patients' information about Peripheral Artery Disease
References
- Song P, et al. Lancet Glob Health. 2019; 7(8): e1020-30.
- Norgren L, et al. J Vasc Surg. 2007; 45(1): S5-67.
MAT-2500698 v2.0
Important Safety Information
Hi-Torque™ Guide Wires for PTA

INDICATIONS FOR USE
This Hi-Torque Guide Wire is intended to facilitate the placement of balloon dilatation catheters during percutaneous transluminal angioplasty (PTA), in arteries such as the femoral, popliteal and infra-popliteal arteries. This guide wire may also be used with compatible stent devices during therapeutic procedures.
The guide wire may also be used to reach and cross a target lesion, provide a pathway within the vessel structure, facilitate the substitution of one diagnostic or interventional device for another, and to distinguish the vasculature.
CONTRAINDICATIONS
Not intended for use in the coronary or cerebral vasculature.
WARNINGS
This device is not designed for use with atherectomy devices.
This device is designed and intended for ONE-TIME USE ONLY. Do not resterilize and / or reuse.
Carefully observe the instructions under “Do Not” and “Do” below. Failure to do so may result in vessel trauma, guide wire damage, guide wire tip separation, or stent damage. If resistance is observed at any time, determine the cause under fluoroscopy and take remedial action as needed. Use the most suitable guide wire for the lesion being treated.
Do Not:
• Push, auger, withdraw, or torque a guide wire that meets excessive resistance. • Torque a guide wire if the tip becomes entrapped within the vasculature. • Allow the guide wire tip to remain in a prolapsed condition. • Deploy a stent such that it will entrap the wire between the vessel wall and the stent.
Do:
• Advance or withdraw the guide wire slowly. • Use the radiopaque marker of the interventional device to confirm position. • Examine the tip movement under fluoroscopy before manipulating, moving, or torquing the guide wire. • Observe the wire under fluoroscopy for tip buckling, which is a sign of resistance. • Maintain continuous flush while removing and reinserting the guide wire to prevent air from entering the catheter system. Perform exchanges slowly to prevent air entry and / or trauma. • When reintroducing the guide wire, confirm that the interventional device tip is free within the vessel lumen and that the tip is parallel to the vessel wall. • Use extreme caution when moving a guide wire through a non-endothelialized stent, or through stent struts, into a bifurcated vessel. Use of this technique involves additional patient risks, including the risk that the wire may become caught on the stent strut.
PRECAUTIONS
Guide wires are delicate instruments and should be handled carefully. Prior to use and when possible during the procedure, inspect the guide wire carefully for bends, kinks, or other damage. Do not use damaged guide wires. Using a damaged guide wire may result in vessel damage and / or inaccurate torque response.
Confirm the compatibility of the guide wire diameter with the interventional device before actual use.
Free movement of the guide wire within the interventional device is an important feature of a steerable guide wire system, because it gives the user valuable tactile information. Test the system for any resistance prior to use. Adjust or replace the hemostatic valve with an adjustable valve if it is found to inhibit guide wire movement.
Never attach the torque device to the modified portion of the proximal end of the extendible guide wire; otherwise, guide wire damage may occur, preventing the ability to attach the DOC Guide Wire Extension.
Hi-Torque Guide Wires with Hydrophilic Coating: Avoid abrasion of the hydrophilic coating. Do not withdraw or manipulate the hydrophilic-coated wire through a metal cannula or sharp-edged object.
ADVERSE EVENTS
Potential Adverse Events associated with use of this device may include the following but are not limited to perforation, dissection, occlusion, myocardial infarction, embolism and infection.
MAT-2307869 v1.0
Hi-Torque Command™ 18 Guide Wire for PTA

INDICATIONS FOR USE
This Hi-Torque™ Guide Wire is intended to facilitate the placement of balloon dilatation catheters during percutaneous transluminal angioplasty (PTA), in arteries such as the femoral, popliteal and infra-popliteal arteries.This guide wire may also be used with compatible stent devices during therapeutic procedures.
The guide wire may also be used to reach and cross a target lesion, provide a pathway within the vessel structure, facilitate the substitution of one diagnostic or interventional device for another, and to distinguish the vasculature.
CONTRAINDICATIONS
Not intended for use in the coronary or cerebral vasculature.
WARNINGS
This device is not designed for use with artherectomy devices.
This device is designed and intended for ONE-TIME USE ONLY. Do not resterilize and / or reuse.
Carefully observe the instructions under “Do Not” and “Do” below. Failure to do so may result in vessel trauma, guide wire damage, guide wire tip separation, or stent damage. If resistance is observed at any time, determine the cause under fluoroscopy and take remedial action as needed. Use the most suitable guide wire for the lesion being treated.
Do Not:
- Push, auger, withdraw, or torque a guide wire that meets excessive resistance.
- Torque a guide wire if the tip becomes entrapped within the vasculature.
- Allow the guide wire tip to remain in a prolapsed condition.
- Deploy a stent such that it will entrap the wire between the vessel wall and the stent.
- Advance or withdraw the guide wire slowly.
- Use the radiopaque marker of the interventional device to confirm position.
- Examine the tip movement under fluoroscopy before manipulating, moving, or torquing the guide wire.
- Observe the wire under fluoroscopy for tip buckling, which is a sign of resistance.
- Maintain continuous flush while removing and reinserting the guide wire to prevent air from entering the catheter system. Perform exchanges slowly to prevent air entry and / or trauma.
- When reintroducing the guide wire, confirm that the interventional device tip is free within the vessel lumen and that the tip is parallel to the vessel wall.
- Use extreme caution when moving a guide wire through a non-endothelialized stent, or through stent struts, into a bifurcated vessel. Use of this technique involves additional patient risks, including the risk that the wire may become caught on the stent strut.
PRECAUTIONS
Guide wires are delicate instruments and should be handled carefully. Prior to use and when possible during the procedure, inspect the guide wire carefully for bends, kinks, or other damage. Do not use damaged guide wires. Using a damaged guide wire may result in vessel damage and / or inaccurate torque response.
Confirm the compatibility of the guide wire diameter with the interventional device before actual use.
Free movement of the guide wire within the interventional device is an important feature of a steerable guide wire system, because it gives the user valuable tactile information. Test the system for any resistance prior to use. Adjust or replace the hemostatic valve with an adjustable valve if it is found to inhibit guide wire movement.
Avoid abrasion of the hydrophilic coating.
Do not withdraw or manipulate the hydrophilic-coated wire through a metal cannula or sharp-edged object.
ADVERSE EVENTS (AEs)
Potential Adverse Events associated with use of this device may include the following but are not limited to:
- Abrupt closure
- Allergic reaction (contrast medium, drug, guide wire material)
- Amputation or limb loss
- Aneurysm or pseudoaneurysm in vessel or at vascular access site
- Angina or coronary ischemia, arrhythmia (including premature beats, bradycardia, atrial or ventricular tachycardia, atrial or ventricular fibrillation)
- Arteriovenous fistula
- Bleeding complications requiring transfusion or surgical intervention
- Critical limb ischemia
- Death
- Detachment of a system component
- Embolization (air, tissue, plaque, thrombotic material, device)
- Emergent surgery
- Fever
- Hematoma or hemorrhagic event, with or without surgical repair
- Hypotension / hypertension
- Infection
- Ischemia or infarction not covered under other AEs
- Myocardial infarction
- Occlusion
- Pain (leg, foot, back and / or insertion site)
- Perforation or rupture
- Peripheral nerve injury
- Pulmonary embolism
- Renal failure or insufficiency secondary to contrast medium (with or without treatment including dialysis)
- Restenosis
- Shock
- Stroke
- Thrombosis
- Tissue injury
- Transient ischemic attack
- Venous thromboembolism
- Vessel dissection
- Vessel spasm or recoil
- Worsening claudication
MAT-2111768 v2.0
Hi-Torque™ Steerable
Guide Wire

INDICATIONS
The Hi-Torque™ Steerable Guide Wire is intended for use in angiographic procedures to introduce and position diagnostic and interventional devices within the peripheral vasculature during percutaneous procedures. The wire can be torqued to facilitate navigation through tortuous vessels.
The Hi-Torque™ Steerable Guide Wire is not intended for use in the coronary or neurovasculature.
CONTRAINDICATIONS
The Hi-Torque™ Steerable Guide Wire is not intended for use in the coronary or neurovasculature.
WARNINGS
This device is designed and intended for ONE-TIME USE ONLY. DO NOT RESTERILIZE AND / OR REUSE.
Observe all guide wire movement in the vessels. Before a guide wire is moved or torqued, the tip movement should be examined under fluoroscopy. Do not torque a guide wire without observing corresponding movement of the tip; otherwise, vessel trauma may occur.
Torquing a guide wire against resistance may cause guide wire damage and / or guide wire tip separation. Always advance or withdraw the guide wire slowly. Never push, auger, withdraw, or torque a guide wire which meets resistance. Resistance may be felt and / or observed under fluoroscopy by noting any buckling of the guide wire tip. Determine the cause of resistance under fluoroscopy and take any necessary remedial action.
If the wire tip becomes entrapped within the vasculature, DO NOT TORQUE THE GUIDE WIRE.
Maintain continuous flush while removing and reinserting the guide wire to prevent air from entering the catheter system. Perform all exchanges slowly to prevent air entry and / or trauma. Wipe the wire before all exchanges.
When reintroducing the guide wire, confirm that the interventional device tip is free within the vessel lumen and not against the vessel wall. Failure to do so may result in vessel trauma upon guide wire exit from the device. Use the radiopaque marker of the interventional device to confirm position.
PRECAUTIONS
Guide wires are delicate instruments and should be handled carefully. Prior to use and when possible during the procedure, inspect the guide wire carefully for bends, kinks, or other damage. Do not use damaged wires. Using a damaged wire may result in vessel damage and / or inaccurate torque response.
Confirm the compatibility of the guide wire diameter with the interventional device before actual use.
Free movement of the guide wire within the interventional device is an important feature of a steerable guide wire system because it gives the user valuable tactile information. Test the system for any resistance prior to use. Adjust or replace the hemostatic valve with an adjustable valve if it is found to inhibit guide wire movement.
ADVERSE EVENTS
Potential Adverse Events associated with use of this device may include the following but not limited to perforation, dissection, occlusion, myocardial infarction, embolism and infection.
MAT-2306607 v1.0
Hi-Torque Command™ 14 ST Guide Wire
Hi-Torque Command™ 14 MT Guide Wire

INDICATIONS FOR USE
The Hi-Torque Command™ 14 ST Guide Wire and Hi-Torque Command™ 14 MT Guide Wire are indicated to facilitate the placement of balloon dilatation catheters during percutaneous transluminal angioplasty (PTA), in arteries such as the femoral, popliteal and infra-popliteal arteries. The guide wires may also be used with compatible stent devices during therapeutic procedures.
The guide wires may also be used to reach and cross a target lesion, provide a pathway within the vessel structure, facilitate the substitution of one diagnostic or interventional device for another, and to distinguish the vasculature.
CONTRAINDICATIONS
The Hi-Torque Command™ 14 ST Guide Wire and Hi-Torque Command™ 14 MT Guide Wire are not intended for use in the coronary or cerebral vasculature.
WARNINGS
This device is not designed for use with atherectomy devices. The safety and effectiveness of the use of the Hi-Torque Command™ 14 ST Guide Wire and Hi-Torque Command™ 14 MT Guide Wire with atherectomy devices are not established.
Use the guide wire prior to the “Use-by date” specified on the package. This device is designed and intended for ONE TIME USE ONLY. Do not resterilize and / or reuse. The safety and effectiveness of this device have not been established after being reprocessed for multiple uses.
Use appropriate anticoagulation per standard of care. Use extreme caution and careful judgement in patients for whom anticoagulation is not indicated.
If contrast agents are used, use extreme caution in patients who have had a severe reaction to contrast agents and who cannot be adequately premedicated.
Persons with a known history of allergies to any of the components of this device listed below may develop an allergic reaction to this guide wire: stainless steel, nitinol (nickel-titanium alloy), platinum-nickel alloy, polytetrafluoroethylene (PTFE) coating, silicone-based hydrophobic coating, tungsten polymer sleeve, urethane, polyvinylpyrrolidone (PVP) coating, tin-silver alloy, gold-tin alloy, and epoxy adhesive. Prior to its use on the patient, the patient should be counseled on the materials contained in the device, and a thorough history of allergies must be discussed.
This device has a hydrophilic coating at the distal end of the device and hydrophobic coatings at the proximal end of the device that increases the lubricity of the guide wire surface for lengths as specified in the table below:
| Product | Type of Coating and Coating Length at the Distal End | Type of Coating and Coating Length at the Proximal End | Hydrophobic PTFE Coating Location (Proximal) |
|---|---|---|---|
| Hi-Torque Command 14 ST, 210 cm | PVP Coating (Hydrophilic coating), 10 cm | Microglide Coating (Hydrophobic coating), 182 cm | Under Microglide Coating, 184 cm |
| Hi-Torque Command 14 ST, 300 cm | PVP Coating (Hydrophilic coating), 10 cm | Microglide Coating (Hydrophobic coating), 264.5 cm | Under Microglide Coating, 276 cm |
| Hi-Torque Command 14 MT, 210 cm | PVP Coating (Hydrophilic coating), 25 cm | Microglide Coating (Hydrophobic coating), 167 cm | Under Microglide Coating, 169 cm |
| Hi-Torque Command 14 MT, 300 cm | PVP Coating (Hydrophilic coating), 25 cm | Microglide Coating (Hydrophobic coating), 249.5 cm | Under Microglide Coating, 261 cm |
Refer to section PREPARATION FOR USE for further information on how to prepare and use this device to ensure it performs as intended. Failure to abide by the warnings in this labeling might result in damage to the device coating, which may necessitate intervention or result in serious adverse events.
Carefully observe the instructions under "Do Not" and "Do" below. Failure to do so may result in vessel trauma, guide wire damage, guide wire tip separation, or stent damage. If resistance is observed at any time, determine the cause under fluoroscopy and take remedial action as needed. Use the most suitable guide wire for the lesion being treated.
Do Not:
- Push, auger, withdraw, or torque a guide wire that meets resistance.
- Torque a guide wire if the tip becomes entrapped within the vasculature.
- Deploy a stent such that it will entrap the wire between the vessel wall and the stent.
Do:
- Advance or withdraw the guide wire slowly.
- Use the radiopaque marker of the interventional device to confirm position.
- Examine the tip movement under fluoroscopy before manipulating, moving, or torquing the guide wire.
- Observe the wire under fluoroscopy for tip buckling, which is a sign of resistance.
- Maintain continuous flush while removing and reinserting the guide wire to prevent air from entering the catheter system. Perform exchanges slowly to prevent air entry and / or trauma.
- When reintroducing the guide wire, confirm that the interventional device tip is free within the vessel lumen and that the tip is parallel to the vessel wall.
- Use extreme caution when moving a guide wire through a non-endothelialized stent, or through stent struts, into a bifurcated vessel. Use of this technique involves additional patient risks, including the risk that the wire may become caught on the stent strut.
- Consider that if a secondary wire is placed in a bifurcation branch, this wire may need to be retracted prior to stent deployment because there is additional risk that the secondary wire may become entrapped between the vessel wall and the stent.
PRECAUTIONS
The Hi-Torque Command™ 14 ST and MT Guide Wire Family has distal ends of varying stiffness. Operate these guide wires carefully so as to not injure the blood vessels, observing the information in these instructions. The higher torque performance, stiffer distal ends, and / or higher advancement force may present a higher risk of perforation or injury than a guide wire with a more pliable distal end. Therefore, use the guide wire with the least stiff distal end that will treat the lesion, and use extreme care to minimize the risk of perforation or other damage to blood vessels.
Guide wires are delicate instruments and should be handled carefully. Prior to use and when possible during the procedure, inspect the guide wire carefully for bends, kinks, or other damage. Do not use damaged guide wires. Using a damaged guide wire may result in vessel damage and / or inaccurate torque response.
Confirm the compatibility of the guide wire diameter with the interventional device before actual use.
Refer to the indication on the label and the Instructions for Use to confirm the appropriate vasculature that this guide wire may be used in. Failure to abide by the above recommendation may result in size mismatch of blood vessel and guide wire, which can result in vessel injury, such as, but not limited to, perforation, dissection, rupture, and avulsion.
Free movement of the guide wire within the interventional device is an important feature of a steerable guide wire system because it gives the user valuable tactile information. Test the system for any resistance prior to use. Adjust or replace the hemostatic valve with an adjustable valve if it is found to inhibit guide wire movement.
It is recommended that the user determine the source of resistance, exercise caution when removing the device and / or other components as a unit, and exchange the device for a new one to complete the procedure.
Avoid abrasion of the coating. Do not withdraw or manipulate the coated guide wire through a metal cannula or sharp-edged object. Manipulation, advancement, and / or withdrawal through a metal device may result in destruction and / or separation of the outer coating, which may cause coating material to remain in the vasculature. This in turn may lead to unintended adverse events requiring additional intervention.
Do not soak the device for longer than 4 hours when the device is not in use. Avoid pre-soaking devices for longer than instructed, as this may impact the coating performance.
When wet, a hydrophilic coating increases the lubricity of the guide wire surface.
The coating swells when exposed to aqueous media; however, this does not have any impact on device use.
The integrity and performance of the device coating can be negatively impacted by preparation with incompatible media or solvents. Please take note of the following important recommendations:
- Avoid wiping the device with dry gauze as this may damage the device coating.
- Avoid excessive wiping of the coated devices.
- Avoid using alcohol, antiseptic solutions, or other solvents to pre-treat the device because this may cause unpredictable changes in the coating which could negatively affect the safety and performance of the guide wire.
Attempting to alter the shape of devices by bending, twisting, or similar methods beyond instructed methods may compromise the coating integrity, and that damage to the coating may not always be noticeable to the naked eye.
One or more components of this device may contain the following substance defined as CMR 1B in a concentration above 0.1% weight by weight: Cobalt; Chemical Abstracts Service (CAS) No. 7440-48-4; EC No. 231-158-0. Current scientific evidence supports that medical devices manufactured from stainless steel alloys containing cobalt do not cause an increased risk of cancer or adverse reproductive effects.
The safety and effectiveness of the Hi-Torque Command™ 14 ST Guide Wire and Hi-Torque Command™ 14 MT Guide Wire have not been established in the pediatric population.
ADVERSE EVENTS
Potential adverse events associated with use of this device may include the following but not limited to:
- Allergic reaction or hypersensitivity to latex, contrast agent, anesthesia, device materials, and drug reactions to anticoagulation, or antiplatelet drugs
- Vascular access complications which may require transfusion or vessel repair, including:
- Bleeding (ecchymosis, oozing, hematoma, hemorrhage, retroperitoneal hemorrhage)
- Arteriovenous fistula, pseudoaneurysm, aneurysm, dissection, perforation / rupture, and laceration
- Embolism (air, tissue, plaque, thrombotic material, or device)
- Target artery complications which may require additional intervention, including:
- Total occlusion or abrupt closure
- Arteriovenous fistula, pseudoaneurysm, aneurysm, dissection, perforation / rupture
- Embolism (air, tissue, plaque, thrombotic material, or device)
- Artery or stent thrombosis
- Stenosis or restenosis
- Vessel spasm
- Claudication
- Venous thromboembolism (including pulmonary embolism)
- Hypotension / hypertension
- Peripheral nerve injury, neuropathy
- Other ischemic conditions / infarct
- Tissue / organ ischemia
- Tissue necrosis
- Ulcer
- Acute limb ischemia
- Infection – local and systemic (including post-procedural)
- Abscess
- Sepsis / infection including bacteremia / cellulitis / septicemia
- Contrast-induced renal insufficiency or renal failure
- Death
MAT-2407512 v2.0
Hi-Torque™ Guide Wires for
PTCA, PTA and Stents

Indications for Use
This Hi-Torque guide wire is intended to facilitate the placement of balloon dilatation catheters during percutaneous transluminal coronary angioplasty (PTCA) and percutaneous transluminal angioplasty (PTA). This guide wire may also be used with compatible stent devices during therapeutic procedures.
Contradindications
Not intended for use in the cerebral vasculature or with atherectomy devices.
Warnings
This device is designed and intended for ONE-TIME USE ONLY. Do not resterilize and / or reuse.
Carefully observe the instructions under “Do Not” and “Do” below. Failure to do so may result in vessel trauma, guide wire damage, guide wire tip separation, or stent damage. If resistance is observed at any time, determine the cause under fluoroscopy and take remedial action as needed. Use the most suitable guide wire for the lesion being treated.
Do Not:
- Push, auger, withdraw, or torque a guide wire that meets resistance.
- Torque a guide wire if the tip becomes entrapped within the vasculature.
- Allow the guide wire tip to remain in a prolapsed condition.
Do:
- Advance or withdraw the guide wire slowly.
- Use the radiopaque marker of the interventional device to confirm position.
- Examine the tip movement under fluoroscopy before manipulating, moving, or torquing the guide wire.
- Observe the wire under fluoroscopy for tip buckling, which is a sign of resistance.
- Maintain continuous flush while removing and reinserting the guide wire to prevent air from entering the catheter system. Perform exchanges slowly to prevent air entry and / or trauma.
- When reintroducing the guide wire, confirm that the interventional device tip is free within the vessel lumen and that the tip is parallel to the vessel wall.
- Use extreme caution when moving a guide wire through a non-endothelialized stent, or through stent struts, into a bifurcated vessel. Use of this technique involves additional patient risks, including the risk that the wire may become caught on the stent strut.
- Consider that if a secondary wire is placed in a bifurcation branch, this wire may need to be retracted prior to stent deployment, because there is additional risk that the secondary wire may become entrapped between the vessel wall and the stent.
For Winn™ family only: The Winn™ family of guide wires have distal ends of varying stiffness. Operate these guide wires carefully so as not to injure the blood vessel, observing the information in these instructions. The higher torque performance, stiffer distal ends, and / or higher advancement force may present a higher risk of perforation or injury than a guide wire with a more pliable distal end. Therefore, use the guide wire with the least stiff distal end that will treat the lesion, and use extreme care to minimize the risk of perforation or other damage to blood vessels.
Precautions
Guide wires are delicate instruments and should be handled carefully. Prior to use and when possible during the procedure, inspect the guide wire carefully for bends, kinks, or other damage. Do not use damaged guide wires. Using a damaged guide wire may result in vessel damage and / or inaccurate torque response.
Confirm the compatibility of the guide wire diameter with the interventional device before actual use.
Free movement of the guide wire within the interventional device is an important feature of a steerable guide wire system, because it gives the user valuable tactile information. Test the system for any resistance prior to use. Adjust or replace the hemostatic valve with an adjustable valve if it is found to inhibit guide wire movement.
Never attach the torque device to the modified portion of the proximal end of the extendible guide wire; otherwise, guide wire damage may occur, preventing the ability to attach the DOC™ Guide Wire Extension.
Hi-Torque Guide Wires with Hydrophilic Coating: Avoid abrasion of the hydrophilic coating. Do not withdraw or manipulate the hydrophilic-coated wire through a metal cannula or sharp-edged object.
Adverse Events
Potential Adverse Events associated with use of this device may include the following but not limited to perforation, dissection, occlusion, myocardial infarction, embolism and infection.
MAT-2306605 v1.0
Hi-Torque™ Guide Wires

Indications for Use
Hi-Torque guide wires are indicated to facilitate the placement of percutaneous devices during Percutaneous Transluminal Angioplasty (PTA) in peripheral arteries such as femoral, popliteal and infra-popliteal arteries. This guide wire may also be used with compatible stent devices during therapeutic procedures.
Contraindications
Hi-Torque wires are not intended for use in the coronary and cerebral vasculature or in patients judged not acceptable for percutaneous intervention.
Warning
A guide wire is a delicate instrument and must not be advanced, withdrawn, or torqued if resistance is met. Guide wire manipulations must always be observed under fluoroscopy.
The Hi-Torque family of guide wires has distal ends of varying stiffness. Operate these guide wires carefully so as to not injure the blood vessel, observing the information in these instructions. The higher torque performance, stiffer distal ends, and / or higher advancement force may present a higher risk of perforation or injury than a guide wire with a more pliable distal end. Therefore, use the guide wire with the least stiff distal end that will treat the lesion, and use extreme care to minimize the risk of perforation or other damage to blood vessels.
If the guide wire is removed and is to be re- inserted, it must be inspected for signs of damage (weakened or kinked segments) prior to re-introduction. Do not re- introduce if guide wire is weakened or kinked.
Do Not:
- Push, auger, withdraw, or torque a guide wire that meets excessive resistance.
- Torque a guide wire if the tip becomes entrapped within the vasculature.
- Allow the guide wire tip to remain in a prolapsed condition.
Do:
- Advance or withdraw the guide wire slowly.
- Use the radiopaque marker of the interventional device to confirm position.
- Examine the tip movement under fluoroscopy before manipulating, moving, or torquing the guide wire.
- Observe the wire under fluoroscopy for tip buckling, which is a sign of resistance.
- Maintain continuous flush while removing and reinserting the guide wire to prevent air from entering the catheter system. Perform exchanges slowly to prevent air entry and / or trauma.
- When reintroducing the guide wire, confirm that the interventional device tip is free within the vessel lumen and that the tip is parallel to the vessel wall.
- Use extreme caution when moving a guide wire through a non-endothelialized stent, or through stent struts, into a bifurcated vessel. Use of this technique involves additional patient risks, including the risk that the wire may become caught on the stent strut.
Precautions
- Failure to follow the instructions may compromise guide wire performance and result in complications.
- Prior to use, confirm compatibility of guide wire outer diameter with the balloon catheter.
- Guide wire advancement, withdrawal, and torquing should be monitored by fluoroscopy.
MAT-2307413 v1.0
Hi-Torque Guide Wires

All Hi-Torque Guide Wires are intended to facilitate the placement of balloon dilatation catheters during percutaneous transluminal coronary angioplasty (PTCA) and percutaneous transluminal angioplasty (PTA).
CONTRAINDICATIONS
Hi-Torque Guide Wires are not intended for use in the cerebral vasculature.
WARNINGS
This device is designed and intended for ONE-TIME USE ONLY. DO NOT RESTERILIZE AND / OR REUSE.
Observe guide wire movement in the vessels. Before a guide wire is moved or torqued, the tip movement should be examined under fluoroscopy. Do not torque a guide wire without observing corresponding movement of the tip; otherwise, vessel trauma may occur. In addition, during catheter manipulations, ensure that the distal guide wire tip is visible.
Torquing a guide wire against resistance may cause guide wire damage and / or guide wire tip separation. Always advance or withdraw the guide wire slowly. Never push, auger, withdraw or torque a guide wire, which meets resistance. Resistance may be felt and / or observed under fluoroscopy by noting any buckling of the guide wire tip. If guide wire tip prolapse is observed or used for positioning, do not allow the tip to remain in a prolapsed condition; otherwise, damage to the guide wire may occur. Determine the cause of resistance under fluoroscopy and take any necessary remedial action.
If the guide wire tip becomes entrapped within the vasculature, DO NOT TORQUE THE GUIDE WIRE.
Maintain continuous flush while removing and reinserting the guide wire to prevent air from entering the catheter system. Perform exchanges slowly to prevent air entry and / or trauma.
When reintroducing the guide wire, confirm that the interventional device tip is free within the vessel lumen and not against the vessel wall. Failure to do so may result in vessel trauma upon guide wire exit of the device. Use the radiopaque marker of the interventional device to confirm position.
PRECAUTIONS
Guide wires are delicate instruments and should be handled carefully. Prior to use and when possible during the procedure, inspect the guide wire carefully for bends, kinks, or other damage. Do not use damaged guide wires. Using a damaged guide wire may result in vessel damage and / or inaccurate torque response.
Confirm the compatibility of the guide wire diameter with the interventional device before actual use.
Free movement of the guide wire within the interventional device is an important feature of a steerable guide wire system because it gives the user valuable tactile information. Test the system for any resistance prior to use. Adjust or replace the hemostatic valve with an adjustable valve if it is found to inhibit guide wire movement.
Never attach the torque device to the modified portion of the proximal end of the extendable guide wire; otherwise, guide wire damage may occur, preventing the ability to attach the DOC™ Guide Wire Extension.
Hi-Torque Guide Wires with Hydrophilic Coating: Avoid abrasion of the hydrophilic coating. Do not withdraw or manipulate the hydrophilic-coated wire in a metal cannula or sharp-edged object.
ADVERSE EVENTS
Potential Adverse Events associated with use of this device may include the following but not limited to perforation, dissection, occlusion, myocardial infarction, embolism and infection.
MAT-2306606 v1.0
Hi-Torque™ Steerable Guide Wires
Hi-Torque Supra Core™ 35 Guide Wire

Intended Use
Hi-Torque Supra Core™ 35 Guide Wires are intended to facilitate the placement and exchange of interventional devices during diagnostic or therapeutic interventional procedures.
Indications
Refer to the device label for any additional product specific indications which may apply.
Contraindications
The Hi-Torque Supra Core™ 35 Guide Wire is not intended for use in the cerebral vasculature. Refer to the device label for any additional product specific contraindications which may apply.
Warnings
This device is designed and intended for ONE TIME USE ONLY. DO NOT RESTERILIZE AND / OR REUSE. Observe all guide wire movement in the vessels. Before a guide wire is moved or torqued, the tip movement should be examined under fluoroscopy. Do not torque a guide wire without observing corresponding movement of the tip; otherwise, vessel trauma may occur.
Torquing a guide wire against resistance may cause guide wire damage and / or guide wire tip separation. Always advance or withdraw the guide wire slowly. Never push, auger, withdraw or torque a guide wire which meets resistance. Resistance may be felt and / or observed under fluoroscopy by noting any buckling of the guide wire tip. Determine the cause of resistance under fluoroscopy and take any necessary remedial action.
If the wire tip becomes entrapped within the vasculature, DO NOT TORQUE THE GUIDE WIRE.
Maintain continuous flush while removing and reinserting the guide wire to prevent air from entering the catheter system. Perform all exchanges slowly to prevent air entry and / or trauma. Wipe the wire before all exchanges.
When reintroducing the guide wire, confirm that the interventional device tip is free within the vessel lumen and not against the vessel wall. Failure to do so may result in vessel trauma upon guide wire exit of the device. Use the radiopaque marker of the interventional device to confirm position.
Precautions
Guide wires are delicate instruments and should be handled carefully. Prior to use and when possible during the procedure, inspect the guide wire carefully for bends, kinks, or other damage. Do not use damaged wires. Using a damaged wire may result in vessel damage and / or inaccurate torque response.
Confirm the compatibility of the guide wire diameter with the interventional device before actual use.
Free movement of the guide wire within the interventional device is an important feature of a steerable guide wire system because it gives the user valuable tactile information. Test the system for any resistance prior to use. Adjust or replace the hemostatic valve with an adjustable valve if it is found to inhibit guide wire movement.
Adverse Events
Potential Adverse Events associated with use of this device may include the following but not limited to perforation, dissection, occlusion, myocardial infarction, embolism, and infection.
MAT-2306103 v1.0
Armada™ 14
PTA Catheter

Indications
The device is indicated to dilate stenoses in femoral, popliteal, infra popliteal and renal arteries and for the treatment of obstructive lesions of native or synthetic arteriovenous dialysis fistulae. The 2.0 to 4.0 mm balloon diameters are also indicated for post-dilatation of balloon-expandable stents up to 40 mm and self-expanding stents up to 80 mm in the vessels listed above.
Contraindications
- Inability to cross lesion with a guide wire
- Use in the coronary arteries
Warnings / Precautions
- This device should only be used by physicians who are experienced and have a thorough understanding of the clinical and technical aspects of PTA.
- One-time use only – do not resterilize! This single use device cannot be reused on another patient, as it is not designed to perform as intended after the first usage. Changes in mechanical, physical, and/or chemical characteristics introduced under conditions of repeated use, cleaning, and/or resterilization may compromise the integrity of the design and/or materials, leading to contamination due to narrow gaps and/or spaces and diminished safety and/or performance of the device. Absence of original labeling may lead to misuse and eliminate traceability. Absence of original packaging may lead to device damage, loss of sterility, and risk of injury to patient and/or user.
- Do not use if inner package is damaged or opened.
- Employ aseptic techniques during removal from the package and during use.
- Any use for procedures other than those indicated in these instructions is not recommended.
- Use prior to the use by date.
- Carefully inspect the catheter prior to use to verify that it has not been damaged during shipment and that its size, shape and condition are suitable for the procedure for which it is to be used.
- Precautions to prevent or reduce blood clotting should be taken when any catheter is used.
- Flush or rinse all products entering the vascular system with sterile isotonic saline or a similar solution via the guide wire access port prior to use. Consider the use of systemic heparinization.
- When the system is introduced into the vascular system, it should be manipulated only under high quality fluoroscopy.
- The Armada™ 14 PTA Catheter must always be introduced, moved and or withdrawn over a guide wire (max. 0.014″).
- Never attempt to move the guide wire when the balloon is inflated.
- Never use air or any gaseous medium to inflate the balloon.
- Do not advance the Armada™ 14 PTA Catheter against significant resistance. The cause of resistance should be determined via fluoroscopy and remedial action taken.
- The minimal acceptable sheath French size is printed on the package label. Do not attempt to pass the Armada™ 14 PTA Catheter through a smaller sized sheath introducer than indicated on the label.
- The size of the inflated balloon should be selected not to exceed the diameter of the artery immediately distal, or proximal, to the stenosis.
- Inflation in excess of the rated burst pressure may cause the balloon to rupture. Use of a pressure monitoring device is recommended.
- If a distal protection device is used, follow the manufacturer’s instruction for use. Allow and maintain adequate distance between the Armada™ 14 PTA Catheter and the distal protection device to avoid engagement.
- Rated burst pressure and balloon fatigue testing of the Armada™ 14 PTA balloons within deployed stents has demonstrated the following:
- The 2.0 to 4.0 mm balloon diameters can safely post-dilate balloon expandable stents up to 40 mm in length.
- The 2.0 to 4.0 mm balloon diameters can safely post-dilate self-expanding stents up to 80 mm in length.
The safety of using additional balloon diameters and/or lengths to post dilate stents has not been established.
- When post-dilating stents, use a balloon length that is appropriate for the deployed stent length.
Potential Complications
The following complications may occur as a result of PTA, but may not be limited to:
- Abrupt closure
- Access site hematoma
- Aneurysm
- Angina
- Arrhythmias
- Arteriovenous fistula
- Bleeding complications which may require transfusion
- Cerebral ischemia/transient ischemic attack (TIA)
- Death
- Embolism (air, tissue, thrombotic, systemic or device component)
- Fever/pyrogenic reaction
- Hypersensitivity or allergic reaction to contrast agents and drug reactions
- Hypertension / hypotension
- Infection
- Ischemia, including tissue ischemia, steal syndrome and necrosis
- Leg edema
- Myocardial ischemia or infarction
- Nausea and vomiting
- Neuropathies or nerve injury
- Occlusion
- Organ failure (single, multiple)
- Pain
- Palpitations
- Pseudoaneurysm
- Renal failure/insufficiency
- Restenosis
- Stroke / cerebrovascular accident (CVA)
- Vascular complications, including entry site, which may require vessel repair
- Vascular thrombosis
- Vessel injury, e.g. dissection, perforation
- Vessel spasm
MAT-2114592 v2.0
Armada™ 14 XT
Percutaneous Transluminal Angioplasty (PTA) Catheter

Indications
The Armada™ 14 XT PTA Catheter is indicated to dilate stenosis in femoral, popliteal, infrapopliteal and renal arteries and for the treatment of obstructive lesions of native or synthetic arteriovenous dialysis fistulae. The 2.0 mm to 5.0 mm balloon diameters are also indicated for post-dilatation of stents in the peripheral vasculature.
Contraindications
The Armada™ 14 XT PTA Catheter is contraindicated for:
- Inability to cross lesion with a guide wire
Warnings
This device is intended for one time use only. DO NOT resterilize and / or reuse it, as this can compromise device performance and increase the risk of cross contamination due to inappropriate reprocessing.
Any use for procedures other than those indicated in these instructions is not recommended.
Precautions to prevent or reduce clotting should be taken when any catheter is used.
The size of the inflated balloon should be selected not to exceed the diameter of the artery immediately distal, or proximal, to the stenosis.
Balloon pressure should not exceed the rated burst pressure (RBP). The RBP is based on results of in vitro testing. At least 99.9% of the balloons (with a 95% confidence) will not burst at or below their RBP. Use of a pressure-monitoring device is recommended to prevent over- pressurization.
To reduce the potential for vessel damage, the inflated diameter of the balloon should approximate the diameter of the vessel just proximal and distal to the stenosis.
Do not use, or attempt to straighten, a catheter if the shaft has become bent or kinked; this may result in the shaft breaking. Instead, prepare a new catheter.
Do not torque the catheter more than one (1) full turn.
If a distal protection device is used, follow the manufacturer’s instruction for use. Allow and maintain adequate distance between the Armada™ 14 XT PTA Catheter and the distal protection device to avoid engagement.
Use only the recommended balloon inflation medium. Never use air or any gaseous medium to inflate the balloon.
When the catheter is exposed to the vascular system, it should be manipulated while under high quality fluoroscopic observation. Do not advance or retract the catheter unless the balloon is fully deflated under vacuum. If resistance is met during manipulation, determine the cause of resistance before proceeding.
Treatment of moderately or heavily calcified lesions increases the risk of acute closure, vessel trauma, balloon burst, balloon entrapment, and associated complications. If resistance is felt, determine the cause before proceeding. Continuing to advance or retract the catheter while under resistance may result in damage to the vessels and / or damage / separation of the catheter.
In the event of catheter damage / separation, recovery of any portion should be performed based on physician determination of individual patient condition and appropriate retrieval protocol.
In cases of extreme vessel tortuosity, it may be necessary to reposition the catheter in a straight segment of the vessel in order to allow guide wire exchange. Do not continue to use a catheter if excessive resistance is felt during guide wire exchanges. Instead, prepare a new catheter.
Precautions
This device should only be used by physicians who are experienced and have a thorough understanding of the clinical and technical aspects of PTA.
Note the “Use by” date specified on the package.
Inspect all product prior to use. Do not use if the package is open or damaged.
Prior to angioplasty, the PTA catheter should be examined to verify functionality and ensure that its size is suitable for the specific procedure for which is to be used.
Precautions to prevent or reduce clotting should be taken when any catheter is used.
Flush or rinse all products entering the vascular system with sterile heparinized normal saline or a similar solution via the guide wire access port prior to use. Consider the use of systemic heparinization.
Never attempt to move the guide wire when the balloon is inflated.
The minimal acceptable sheath / guiding catheter French size is printed on the package label. Do not attempt to pass the Armada™ 14 XT PTA Catheter through a smaller sized sheath / guiding catheter than indicated on the label.
If the surface of the Armada™ 14 XT PTA Catheter becomes dry, wetting with heparinized normal saline will reactivate the coating.
Do not reinsert the Armada™ 14 XT PTA Catheter into the coil dispenser after procedural use.
Bench testing was conducted with 0.014" (0.36 mm) constant diameter guide wires to establish guide wire compatibility. If another type of guide wire is selected with a different dimensional profile, the compatibility (e.g., wire resistance) should be considered prior to use.
The safety and effectiveness of this PTA balloon catheter for the treatment of in-stent restenosis (ISR) have not been established.
Adverse Events
Possible adverse effects include, but are not limited to, the following:
- Abrupt closure
- Access site hematoma
- Aneurysm
- Angina
- Arrhythmias
- Arteriovenous fistula
- Bleeding complications which may require transfusion
- Cerebral ischemia / transient ischemic attack (TIA)
- Death
- Embolism (air, tissue, thrombotic, systemic or device component)
- Fever/pyrogenic reaction
- Hypersensitivity or allergic reaction to contrast agents and drug reactions
- Hypertension / hypotension
- Infection
- Ischemia, including tissue ischemia, steal syndrome and necrosis
- Leg edema
- Myocardial ischemia or infarction
- Nausea and vomiting
- Neuropathies or nerve injury
- Occlusion
- Organ failure (single, multiple)
- Pain
- Palpitations
- Pseudoaneurysm
- Renal failure / insufficiency
- Restenosis
- Stroke / cerebrovascular accident (CVA)
- Vascular complications, including entry site, which may require vessel repair
- Vascular thrombosis
- Vessel injury, e.g. dissection, perforation
- Vessel spasm
MAT-2114593 v2.0
Armada™ 18 Percutaneous Transluminal Angioplasty (PTA) Catheter

Indications
The Armada™ 18 is indicated to dilate stenosis in femoral, popliteal, infra-popliteal, and renal arteries and for the treatment of obstructive lesions of native or synthetic arteriovenous dialysis fistulae. In addition, the device is also indicated for post-dilatation of balloon expandable and self-expanding stents.
Contraindications
- Inability to cross lesion with a guide wire
- Use in the coronary arteries
Warnings / Precautions
- This device should only be used by physicians who are experienced and have a thorough understanding of the clinical and technical aspects of PTA.
- One-time use only – do not resterilize! This single use device cannot be reused on another patient, as it is not designed to perform as intended after the first usage. Changes in mechanical, physical, and / or chemical characteristics introduced under conditions of repeated use, cleaning, and/ or resterilization may compromise the integrity of the design and / or materials, leading to contamination due to narrow gaps and / or spaces and diminished safety and / or performance of the device. Absence of original labeling may lead to misuse and eliminate traceability. Absence of original packaging may lead to device damage, loss of sterility, and risk of injury to patient and / or user.
- Do not use if inner package is damaged or opened.
- Employ aseptic techniques during removal from the package and during use.
- Any use for procedures other than those indicated in these instructions is not recommended.
- Use prior to the use by date.
- Carefully inspect the catheter prior to use to verify that it has not been damaged during shipment and that its size, shape and condition are suitable for the procedure for which it is to be used.
- Precautions to prevent or reduce blood clotting should be taken when any catheter is used.
- Flush or rinse all products entering the vascular system with sterile isotonic saline or a similar solution via the guide wire access port prior to use. Consider the use of systemic heparinization.
- When the system is introduced into the vascular system, it should be manipulated only under high quality fluoroscopy.
- The Armada™ 18 PTA Catheter must always be introduced, moved and or withdrawn over a guide wire (max. 0.018″).
- Never attempt to move the guide wire when the balloon is inflated.
- Never use air or any gaseous medium to inflate the balloon.
- Do not advance the Armada™ 18 PTA Catheter against significant resistance. The cause of resistance should be determined via fluoroscopy and remedial action taken.
- The minimal acceptable sheath French size is printed on the package label. Do not attempt to pass the Armada™ 18 PTA Catheter through a smaller sized sheath introducer than indicated on the label.
- The size of the inflated balloon should be selected not to exceed the diameter of the artery immediately distal, or proximal, to the stenosis.
- Inflation in excess of the rated burst pressure may cause the balloon to rupture. Use of a pressure monitoring device is recommended.
- If a distal protection device is used, follow the manufacturer’s instruction for use. Allow and maintain adequate distance between the Armada™ 18 PTA Catheter and the distal protection device to avoid engagement.
- Rated burst pressure and balloon fatigue testing of the Armada™ 18 PTA balloons within deployed stents has demonstrated that the Armada™ 18 can safely post-dilate balloon expandable and self-expanding stents.
- When post-dilating stents, use a balloon length that is appropriate for the deployed stent length.
Potential Complications
The following complications may occur as a result of PTA, but may not be limited to:
- Abrupt closure
- Allergic reaction (contrast medium, drug, or stent material)
- Aneurysm, pseudoaneurysm or arteriovenous fistula
- Angina or coronary ischemia
- Arrhythmias (including premature beats, bradycardia, atrial or ventricle tachycardia, atrial or ventricular fibrillation)
- Bleeding complications requiring transfusion or surgical intervention
- Death
- Detachment and / or implantation of a component of the system
- Embolization, arterial or other (air, tissue, plaque, thrombotic material, device)
- Emergent or urgent surgery
- Fever
- Hematoma or hemorrhagic event with or without surgical repair
- Hyperperfusion syndrome
- Hypotension or hypertension
- Infection
- Ischemia or infarction of tissue or organ not covered under other adverse events
- Myocardial infarction
- Pain (limb or catheter site)
- Peripheral nerve injury
- Pulmonary embolism
- Renal failure or insufficiency
- Restenosis of vessel
- Shock
- Stroke, cerebrovascular accident (CVA), or transient ischemic attack (TIA)
- Target limb loss (amputation of toe, foot, and / or leg)
- Vascular thrombosis or occlusion at puncture site, treatment site, or remote site
- Venous thromboembolism
- Vessel dissection, perforation, or rupture
- Vessel spasm or recoil
- Worsening claudication or rest pain
MAT-2114595 v2.0
JADE‡ Rx, JADE‡ 014, JADE‡ 018, and JADE‡ 035
PTA Balloon Dilatation Catheters

INDICATIONS
The JADE‡ PTA Balloon Dilation Catheter is indicated for Percutaneous Transluminal Angioplasty in the peripheral vasculature, including iliac, femoral, iliofemoral, popliteal, infra-popliteal, and renal arteries, and for the treatment of obstructive lesions of native or synthetic arteriovenous dialysis fistulae. This device is also indicated for post-dilation of balloon expandable and self-expanding stents in the peripheral vasculature.
CONTRAINDICATIONS
The use of the JADE‡ PTA Balloon Dilatation Catheter is contraindicated:
- For use in the coronary or neuro vasculature.
- Where there is the inability to cross the target lesion with a guidewire.
WARNINGS
When using this type of device, the following warnings should be observed:
- This device is intended for single use only. Do not resterilize and/or reuse, as this can potentially result in compromised device performance and increased risk of cross-contamination.
- When the catheter is exposed to the vascular system, it should be manipulated while under high-quality fluoroscopic observation. Do not advance or retract the catheter unless the balloon is fully deflated under vacuum. If resistance is met during manipulation, determine the cause of the resistance before proceeding. Applying excessive force to the catheter can result in separation of the tip or balloon.
- To reduce the potential for vessel damage, the inflated diameter of the balloon should approximate the diameter of the vessel just proximal and distal to the stenosis.
- Balloon pressure should not exceed the rated burst pressure (RBP) indicated on the package. The rated burst pressure is based on the results of in vitro testing. At least 99.9 percent of the balloons, (with at least 95 percent confidence) will not burst at or below the rated burst pressure. Use of a pressure monitoring device is recommended to prevent over pressurization.
- To reduce the potential for air embolus into the vessel, use only the recommended balloon inflation medium. Never use air or any gaseous medium to inflate the balloon.
- For the rapid exchange catheters, do not re-straighten a kinked hypotube; straightening a kinked metal shaft may result in breakage of the shaft.
PRECAUTIONS
- The catheter system should be used only by physicians trained in percutaneous transluminal angioplasty.
- Use the catheter prior to the “Use By" date specified on the package.
- Prior to angioplasty, the catheter should be examined to verify functionality and ensure that its size and shape are suitable for the specific procedure for which it is to be used.
- Do not use oil-based contrast medium, organic solvents or alcohols; there is a possibility of catheter leak, damage or lubrication loss.
- The balloon deflation time has been established as 30 seconds (for 0.014” Rx) and 60 seconds (for 0.014”, 0.018”, and 0.035” OTW catheters) based on in vitro bench testing results.
- Use with caution for procedures involving calcified lesions or synthetic vascular grafts due to the abrasive nature of these lesions.
- Do not reinsert the PTA catheter into the coil dispenser after procedural use.
- Discard all disposable devices used during this procedure per local requirements for medical device waste disposal.
ADVERSE EFFECTS
Adverse effects due to the use of this product include, but are not limited to, the following:
- Acute or subacute thrombosis
- Acute vessel closure
- Allergic reaction to device, contrast medium, or medication
- Aneurysm
- Arrhythmias
- Arteriovenous fistula
- Death
- Dissection (perforation, rupture, or injury) of the vessel
- Hemorrhage or hematoma
- Hypertension
- Hypotension
- Infection
- Occlusion of the artery
- Restenosis of the dilated vessel
- Stroke, air embolism and embolization of fragmentation of thrombotic or atherosclerotic material
MAT-2400998 v2.0
Emboshield NAV6 ™ Embolic Protection System

Indications
The Emboshield NAV6 ™ Embolic Protection System is indicated for use as a guide wire and embolic protection system to contain and remove embolic material (thrombus / debris) while performing angioplasty and stenting procedures in carotid arteries and while performing atherectomy, during standalone procedures or together with PTA and/or stenting, in lower extremity arteries. The diameter of the artery at the site of the Filtration Element placement should be between 2.5 and 7.0 mm.
Contraindications
The Emboshield NAV6 ™ Embolic Protection System is contraindicated for use in
- Patients in whom anticoagulant and / or antiplatelet therapy is contraindicated.
- Patients with severe vascular tortuosity or anatomy that would preclude the safe introduction of the Guiding Catheter / Introducer Sheath, Embolic Protection System.
- Patients with a known allergy or hypersensitivity to device materials (Nitinol, Nickel, Titanium) or contrast medium, who cannot be adequately premedicated.
- Patients with uncorrected bleeding disorders.
- Lesions in the ostium of the common carotid artery.
- Inability to cross the lesion with the BareWire™ Filter Delivery Wire.
- Diffusely diseased vessels where there is no disease-free section in which to deploy the Filtration Element
- Insufficient straight section of vessel distal to the lesion to permit Filtration Element deployment.
Warnings
Use of the device should be restricted to physicians trained to the specifics of the device and to the Instructions for Use. Operators must be knowledgeable of the current medical literature and familiar with the principles, clinical applications, complications, side effects and hazards commonly associated with carotid and lower extremity interventional procedures.
General Warning
Refer to instructions supplied with all interventional devices to be used with the Emboshield NAV6 ™ Embolic Protection System for their intended uses, contraindications, and potential complications.
The Emboshield NAV6 ™ System is supplied sterile. Do not use if the package has been opened or is damaged. Carefully inspect the system components prior to use to verify that they have not been damaged and that the size, shape and condition are suitable for the procedure for which they are to be used. A device or access device that is kinked or damaged in any way should not be used.
Safety and effectiveness of this device as an embolic protection system has not been established in the coronary or cerebral vasculature.
The safety and efficacy of the Emboshield NAV6 ™ Embolic Protection System has not been demonstrated with carotid stent systems other than the Xact™ or Acculink™ Carotid Stent Systems.
The safety and efficacy of the Emboshield NAV6 ™ Embolic Protection System has not been demonstrated with atherectomy devices other than Turbo-Elite‡ Laser Atherectomy Catheter, Jetstream‡ Single Cutter (SC) Atherectomy Catheter, Jetstream‡ eXpandable Cutter (XC) Atherectomy Catheter and TurboHawk‡ Peripheral Plaque Excision System.
The Emboshield NAV6 ™ device can only be used with the BareWire™ Filter Delivery Wire. Use of the device with any guide wire other than the BareWire™ Filter Delivery Wire will lead to loss of the Filtration Element during the procedure or an inability to retrieve the Filtration Element.
This device is designed and intended for single use only. Do not reuse, reprocess or resterilize. Reuse, reprocessing or resterilization may compromise the structural integrity of the device and / or delivery system and / or lead to device failure, which may result in patient injury, illness or death. Reuse, reprocessing or resterilization may also create a risk of contamination of the device and / or cause patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device and / or delivery system may lead to injury, illness or death of the patient.
Store in a cool, dark, dry area. Do not use the product after the Use by date specified on the label.
Overstretching of the artery may result in rupture and life-threatening bleeding.
Appropriate antiplatelet, anticoagulant and, if necessary, vasodilator therapy must be used during the procedure. Anticoagulant therapy sufficient to maintain an Activated Clotting Time of at least 250 seconds for the duration of the procedure is recommended.
Adequate guide catheter or sheath support is required for the introduction of the RX Delivery Catheter, RX Retrieval Catheter, and all interventional devices to be used during the procedure.
Maintain a snug seal between the device and the hemostasis valve during catheter insertion. Failure to observe this may result in air being drawn into the access device through the hemostasis valve. Device insertion should be performed slowly to minimize the risk of air entrainment. It is therefore recommended that flushing of contrast media (or other fluids) is performed before or after insertion of the catheter, but not while the catheter is within the access device.
Do not advance any component of the Emboshield NAV6 ™ Embolic Protection System against significant resistance. The cause of any resistance should be determined via fluoroscopy and remedial action taken.
Torquing the BareWire™ Filter Delivery Wire against resistance may cause BareWire™ Filter Delivery Wire damage and / or BareWire™ Filter Delivery Wire tip separation.
Perform all exchanges slowly to prevent air embolism or trauma to the artery.
The RX Delivery Catheter should not be pulled back quickly in the event that resistance is felt during catheter advancement. Rapid withdrawal of the RX Delivery Catheter in the presence of resistance may result in premature deployment of the Filtration Element.
In the event of complications, surgical intervention may be required.
A high pressure contrast injection may cause Filtration Element movement in the vessel.
Do not attempt to move the Filtration Element after deployment and prior to the start of retrieval. Do not torque the RX Delivery and RX Retrieval Catheters.
Allow and maintain adequate distance between the Emboshield NAV6 ™ Filtration Element and other interventional devices (including carotid stent systems and balloons) to avoid engagement.
When introducing the delivery system, confirm that the wire tip is free within the vessel lumen and is not directed into the vessel wall. Failure to do so may result in vessel trauma. Use the radiopaque marker on the interventional device to confirm position.
Avoid excessive movement of the filter basket during catheter device exchanges. Excessive movement of the deployed basket may cause vessel trauma or spasm.
After use, this device, its accessories and packaging should be appropriately classified for disposal (e.g., biohazard, sharps, non-hazardous waste, etc.) and carefully disposed of in compliance with facility procedures and applicable laws and regulations.
The following vessel anatomy specifications preclude the use of the stent system or appropriate positioning of the embolic protection system:
- Hemoglobin (Hgb) < 8 gm/dl (unless on dialysis), platelet count < 50,000, INR > 1.5 (irreversible), or heparin-associated thrombocytopenia.
- Evidence of a stroke within the previous 30 days.
- History of ipsilateral stroke with fluctuating neurologic symptoms within 1 year.
- Patients with total occlusion of the target vessel.
- Patients with evidence of intraluminal thrombus thought to increase the risk of plaque fragmentation and distal embolization.
- Any condition that precluded proper angiographic assessment or made percutaneous arterial access unsafe (e.g., morbid obesity, sustained systolic blood pressure > 180 mmHg).
- Known cardiac sources of emboli.
- Patients with highly calcified lesions resistant to PTA.
- Atherosclerotic disease involving adjoining vessels precluding safe placement of the guiding catheter or sheath.
- The safety and effectiveness of concurrent treatment of lesions in patients with bilateral carotid artery disease have not been established.
Caution should be used if pre-dilating the lesion without embolic protection as this may increase the risk of an adverse outcome.
The BareWire™ Filter Delivery Wire should be kept moistened throughout the procedure.
The Filtration Element can only be positioned proximal to the radiopaque distal section of the Filter Delivery Wire.
To reduce the potential for the liberation of emboli during lesion crossing, the device should be carefully manipulated and not advanced against resistance.
If the Filtration Element moves into the stented vessel segment prior to retrieval, DO NOT RETRIEVE. Use the Retrieval Catheter to gently maneuver the Filtration Element distally until it is situated in an unstented portion of vessel. Retrieval should then proceed.
Maintain proper guiding catheter / sheath support throughout the procedure. Ensure that there is enough distance between the proximal tip of the Filtration Element and the most distal tip of any interventional device to be introduced over the Filter Delivery Wire to avoid engagement. The tip of a balloon catheter or a stent delivery system or an atherectomy device should not contact the Filtration Element. Failure to maintain adequate distance could result in inadvertent Filtration Element movement and Stent Delivery System tip / Filtration Element entanglement and / or Filtration Element /Stent entanglement if guide catheter or sheath prolapse occurs.
In patients requiring the use of antacids and / or H2-antagonists before or immediately after stent placement, oral absorption of antiplatelet agents (e.g., aspirin) may be adversely affected.
The minimum guide catheter or sheath internal diameter is printed on the package label. Do not use a smaller guide catheter or sheath than indicated.
For proper positioning of the filter basket, the vessel distal to the lesion should have an absence of excessive tortuosity and be of adequate length (approximately 4 cm distal to the lesion and proximal to the petrous portion of the vessel).
Do not pull back on the BareWire™ Filter Delivery Wire during advancement.
The Emboshield NAV6 ™ Embolic Protection System is not to be deployed with an access device that uses an integrated leaflet type valve.
Precautions
The device must only be flushed using the 3-ml syringe and flushing tip provided. Use with fixed (passive) hemostatic valves is not recommended.
For best device performance, the guide wire exit notch should remain within the guiding catheter or sheath.
The delivery system is not designed for use with power injection. Use of power injection may adversely affect device performance.
Care must be used when removing the filter basket through a newly deployed stent to maintain filter basket integrity and to avoid disrupting the stent geometry.
To optimize system performance, it is imperative that careful attention is paid to the preparation of the system using the preparation techniques specified in the Instructions For Use. The size of the Filtration Element should be selected to match the diameter of the vessel at the intended site of deployment.
The clinician should take into consideration the slight increase in vessel diameter that may occur after treatment of the lesion.
Precautions to prevent or reduce clotting should be taken when any interventional device is used. Flush or rinse all devices entering the vascular system with heparinized normal saline or alternative anticoagulant, prior to use.
The Emboshield NAV6 ™ Embolic Protection System must be used with a guiding catheter or introducer sheath to maintain adequate support for the BareWire™ Filter Delivery Wire throughout the procedure.
Do not torque the RX Delivery and RX Retrieval Catheters.
The maintenance of blood flow through the device should be observed throughout the procedure by the use of contrast injection.
If excessive debris is collected in the Filtration Element such that distal profusion of dye is significantly reduced or no dye is perfusing past the filter, the Emboshield NAV6 ™ device may have reached its maximum capacity to contain emboli. Remove and replace the
Emboshield NAV6 ™ device. Otherwise, it may be difficult to completely recover all embolic debris and the potential for thrombus formation may increase.
Do not attempt to move the Filtration Element after deployment and prior to the start of retrieval.
Venous access should be available during carotid stenting in order to manage bradycardia and / or hypotension by either pharmaceutical intervention or place of a temporary pacemaker, if needed.
Removal of the BareWire™ Filter Delivery Wire with the Emboshield NAV6 ™ Filtration Element through any interventional devices other than the Emboshield NAV6 ™ RX Retrieval Catheter has not been tested.
The minimum expanded stent internal diameter required for retrieval of a large embolic load is 2.5 mm.
During the insertion of Rapid Exchange catheters through guide catheters or sheaths, careful handling is required to ensure that air is not drawn into the access device. It is therefore recommended that flushing of contrast media (or other fluids) is performed before or after insertion of the catheter, but not while the catheter is within the access device.
Deployment of the Filtration Element, the subsequent deployment of complementary interventional devices over the BareWire™ Filter Delivery Wire, and the retrieval of the Filtration Element should only be performed under fluoroscopic observation.
Potential Adverse Events
As reported in the literature, the following adverse events are potentially associated with carotid stents and embolic protection systems:
- Allergic reaction or hypersensitivity to latex, contrast agent, anesthesia, stent material (Nitinol, Nickel, Titanium) and drug reactions to anticoagulation, or antiplatelet drugs
- Vascular access complications which may require transfusion or vessel repair, including:
- Bleeding (ecchymosis, oozing, hematoma, hemorrhage, retroperitoneal hemorrhage)
- Arteriovenous fistula, pseudoaneurysm, aneurysm, dissection, perforation/rupture, and laceration
- Embolism (air, tissue, plaque, thrombotic material or device)
- Thrombophlebitis
- Target artery complications which may require additional intervention, including:
- Total occlusion or abrupt closure
- Arteriovenous fistula, pseudoaneurysm, aneurysm, dissection, perforation/rupture
- Embolism (air, tissue, plaque, thrombotic material or device)
- Stenosis or restenosis
- Artery, stent, or filter thrombosis / occlusion thrombosis
- Vessel spasm
- Cardiac arrhythmias (including conduction disorders, atrial and ventricular arrhythmias)
- Cardiac ischemic conditions (including myocardial ischemia, myocardial infarction, and unstable or stable angina pectoris)
- Stroke/Cerebrovascular accident (CVA) and Transient Ischemic Attack (TIA)
- System organ failures:
- Cardio Pulmonary failure
- Renal failure / insufficiency
- Blood cell disorders including heparin induce thrombocytopenia and other coagulopathy
- Hypotension/hypertension
- Peripheral nerve injury
- Other ischemic conditions/infarct
- Infection - local and systemic (including postproceural)
- Chest pain
- Edema/Cerebral edema and fluid overload
- Fever
- Pain, including headache
- Hyperperfusion syndrome
- Other neurologic and systemic complications
- Cerebral hemorrhage
- Death
Any adverse event occurring involving the Emboshield NAV6 ™ Embolic Protection System should be reported immediately to Abbott Vascular, Customer Service: 1-800 227-9902.
MAT-2208561 v3.0
Diamondback 360™ Peripheral Orbital Atherectomy System, Diamondback 360™ Peripheral Orbital Atherectomy System – Exchangeable Series

Applies to Diamondback 360™ Peripheral Orbital Atherectomy System only: Including the Orbital Atherectomy Device (OAD) with GlideAssist™, Saline Pump, Viperwire Advance™ Peripheral Guide Wire, and Viperwire Advance™ with Flex Tip Peripheral Guide Wire
Applies to Diamondback 360™ Peripheral Orbital Atherectomy System – Exchangeable Series only: Including the Orbital Atherectomy Device (OAD), Handle, Orbital Atherectomy Cartridge, Saline Pump, Viperwire Advance™ Peripheral Guide Wire, and Viperwire Advance™ with Flex Tip Peripheral Guide Wire
INDICATIONS
The Diamondback 360™ Peripheral Orbital Atherectomy System/Diamondback 360™ Peripheral Orbital Atherectomy System – Exchangeable Series (OAS) is a percutaneous orbital atherectomy system indicated for use as therapy in patients with occlusive atherosclerotic disease in peripheral arteries and who are acceptable candidates for percutaneous transluminal atherectomy.
The OAS Solid, Classic and Micro Crowns support removal of stenotic material from artificial arteriovenous dialysis fistulae (AV shunt). The system is a percutaneous orbital atherectomy system indicated as a therapy in patients with occluded hemodialysis grafts who are acceptable candidates for percutaneous transluminal angioplasty.*
*The 2.00 Max Crown has not been tested to support removal of stenotic material from artificial dialysis fistulae (AV shunt).
CONTRAINDICATIONS
Use of the OAS is contraindicated in the following situations:
- The guide wire cannot be passed across the peripheral lesion.
- The system cannot be used in coronary arteries.
- The target lesion is within a bypass graft or stent.
- The patient has angiographic evidence of thrombus; thrombolytic therapy must be instituted prior to atherectomy.
- The patient has angiographic evidence of significant dissection at the treatment site. The patient may be treated conservatively to permit the dissection to heal before treating the lesion with the OAS.
WARNINGS
- Do not use the OAD in a vessel that is too small for the crown. The reference vessel diameter at the treatment area must be at least 2.00 mm in diameter for the 1.25mm Micro crown.
- If mechanical failure of the OAD occurs before or during the atherectomy procedure, discontinue use immediately. Do not attempt to use a damaged OAD or other system component. Use of damaged components may result in system malfunction or patient injury.
- Do not use the OAD during spasm of the vessel.
- Only use approved and compatible Viperwire Advance™ guide wires. Reference the Instructions for Use for the appropriate guide wire to use based on the OAD shaft configuration. Follow CSI’s instructions related to guide wire use.
- Do not continue treatment if the guide wire or the OAD becomes sub-intimal.
- Immediately stop use if the OAD stalls. Review for complications and mechanical failure if a stall condition occurs. Do not change to a higher speed if the OAD stalls.
- Performing treatment in vessels or bifurcations that are excessively tortuous or angulated may result in vessel damage.
- Always use fluoroscopy when advancing the guide wire to avoid misplacement, dissection, or perforation. The OAD tracks over the guide wire, so it is imperative that the guide wire be initially placed through the stenotic lumen and not in a false channel.
- Do not inject contrast while OAD crown is spinning. OAD failure or patient harm may occur.
- Do not attempt aspiration through the OAD or saline line while placed within the body. If saline is pulled out through the OAD or saline line, air may enter the system.
- If air is noticed in the system while the OAD is within the body, discontinue treatment by pressing the OAS Pump power button and carefully remove the OAD driveshaft and crown from the introducer sheath/guide catheter.
- Handle the OAD and guide wire carefully. A tight loop, kink, or bend in the guide wire may cause damage and system malfunction during use.
- Never operate the OAD without normal saline and lubricant solution. Flowing saline and lubricant solution is required for cooling and lubricating the OAD during use to avoid overheating and permanent damage to the OAD and possible patient injury.
- The crown at the distal tip of the OAD operates at very high speeds. Do not allow body parts or clothing to come into contact with the crown. Physical injury or entanglement may occur.
- Never advance the orbiting crown to the point of contact with the guide wire spring tip. Distal detachment and embolization of the tip may result.
- Always advance the orbiting, abrasive crown by using the crown advancer knob. Never advance the orbiting crown by advancing the shaft or handle. Guide wire buckling may occur, and perforation or vascular trauma may result.
- Always keep the crown advancing or retracting while it is at high rotational speeds. Do not allow the crown to remain in one location for more than 2–3 seconds. Maintaining the crown in one location while it is orbiting at high speeds may lead to excessive tissue removal.
- Do not start or stop orbiting of the crown when tight in a lesion.
- Never force the crown when rotational or translational resistance occurs; vessel perforation may occur. If resistance to motion is noted, retract the crown and stop treatment immediately. Use fluoroscopy to analyze the situation.
- When treating tight stenosis, create a channel at low or medium speed before traversing the lesion at high speed. Crossing a tight stenosis on high speed may cause the shaft and/or guide wire to fracture as a result of excessive force.
- While advancing the crown through the introducer sheath/guide catheter, do not activate crown rotation. The crown must not spin while located within the introducer sheath/guide catheter.
- Do not prolapse or bend the guide wire core. If the spring tip becomes prolapsed, keep the bend/prolapse contained within the spring tip section only. Spinning over a prolapsed or bent guide wire core can result in damage to the guide wire or OAD.
- The system should not be used on children or pregnant women.
- Do not re-use or re-sterilize the OAD. If the OAD is re-used, the OAD may not function as intended and serious infection, leading to potential harm and/or death, may occur.
- Do not spin the crown in GlideAssist™ mode with the guide wire brake lever in the unlocked position, without first securing the guide wire by holding it with fingers or with the guide wire torquer. If using the guide wire torquer, ensure that it is securely fastened to the guide wire before starting to spin the crown. Failure to secure the guide wire when the brake is unlocked could allow the guide wire to spin and/or migrate while in GlideAssist™ mode which may result in patient harm.
PRECAUTIONS
- Do not use the product if the sterile packaging appears damaged or the shelf life has expired.
- User should take precautions when using the OAS or its components in conjunction with other medical equipment
- Do not flip contents of trays into the sterile field as damage may occur. Components within trays must be carefully removed and placed into sterile field to avoid damage.
- Follow standard hospital atherectomy policies and procedures, including those related to anticoagulation and vasodilator therapy.
- Radiographic equipment for fluoroscopy should be used to provide high-resolution images. Guide wires and catheters should only be manipulated under fluoroscopy.
- Because of the torque responsiveness of ViperWire™ peripheral guide wires , they are more difficult to handle than other commercially available guide wires used in peripheral angioplasty. Exercise care when using these guide wires.
- Use only normal saline and lubricant solution as the infusate. (Drugs such as vasodilators are added to the infusate at the physician’s discretion). The OAD may malfunction if contrast or other substances are injected into the OAD infusion port.
- Do not operate the OAD without using recommended ViperSlide™ Lubricant concentration (maximum speeds may not be achieved without lubricants).
- Ensure the OAD strain relief remains straight during atherectomy treatment.
- To relieve compression in the driveshaft, lock the crown advancer knob at 1 cm from the full back position, advance device over the wire to a position proximal from the lesion, deploy the guide wire brake, then unlock the crown advancer knob and move it fully proximal. If the OAD is started with existing compression in the driveshaft, it may result in the crown springing forward.
- If 1:1 motion is not observed between the crown advancer knob and the crown, retract and re-advance the crown into the lesion. Repeat retracting and advancing the crown into the lesion until 1:1 movement is observed. If the knob and the crown are not moving together, the crown may be driven into the lesion with too much force and may result in the crown springing forward on exiting the lesion.
- When moving the crown back and forth across the lesion, employ a series of intermittent treatment intervals and rest periods. 30-second rest periods are recommended after 30-second treatment intervals, with a maximum total treatment time of 8 minutes.
- Monitor the saline fluid level during the procedure. Normal saline and lubricant solution infusion is critical to OAD performance.
- Do not kink or crush the saline tubing. Flow of saline will be reduced.
- Check the saline tubing and connections for leaks during the procedure.
- Do not allow fluid to leak onto electrical connections of the OAS pump.
Applies to Diamondback 360™ Peripheral OAS – Exchangeable Series only:
- Do not detach the cartridge from the handle when the OAD is over the guide wire. Kinking of the guide wire and/or not being able to reconnect the cartridge and the handle may occur.
- Do not track only the cartridge into the patient and then attempt to connect the handle. Kinking of the guide wire and/or not being able to reconnect the cartridge and the handle may occur.
- Do not attempt to load a guide wire with a crossing profile >0.014” through the proximal end of the OAD. Guide wire with a crossing profile >0.014” will not fit through the internal components of the OAD.
- Do not attempt to remove the cartridge from the handle when spinning.
POTENTIAL ADVERSE EVENTS
Potential adverse events that may occur and/or require intervention include, but are not limited to:
- Allergic reaction to medication/media/device components
- Amputation
- Anemia
- Aneurysm
- Bleeding complications which may require transfusion
- Cerebrovascular accident (CVA)
- Death
- Distal embolization
- Entry site complications
- Hemolysis
- Hypotension/hypertension
- Infection
- Myocardial infarction
- Pain
- Pseudoaneurysm
- Restenosis of treated segment that may require revascularization
- Renal insufficiency/failure
- Slow flow or no reflow phenomenon
- Thrombus
- Vessel closure, abrupt
- Vessel injury, including dissection and perforation that may require surgical repair
- Vessel spasm
- Vessel occlusion
Diamondback 360™ Peripheral OAS/Diamondback 360™ Peripheral OAS– Exchangeable Series are manufactured and distributed by Cardiovascular Systems, Inc. (CSI). CSI is a subsidiary of the Abbott Group of Companies.
MAT-2303958 v1.0
Esprit™ BTK Everolimus Eluting Resorbable Scaffold System

Indications
The Esprit™ BTK Everolimus Eluting Resorbable Scaffold System is indicated for improving luminal diameter in infrapopliteal lesions in patients with chronic limb-threatening ischemia (CLTI) and total scaffolding length up to 170 mm with a reference vessel diameter of ≥ 2.5 mm and ≤ 4.00 mm.
Contraindications
The Esprit™ BTK Everolimus Eluting Resorbable Scaffold System is contraindicated for use in:
- Patients who cannot tolerate, including allergy or hypersensitivity to, procedural anticoagulation or the post-procedural antiplatelet regimen.
- Patients with hypersensitivity or contraindication to everolimus or structurally related compounds or known hypersensitivity to scaffold components poly(L-lactide), poly(D, L-lactide), and platinum.
Warnings
- This device is intended for single use only. Do not reuse, reprocess, or re-sterilize. Note the product "Use-by" date on the package. Reuse, reprocessing, or re-sterilization may compromise the structural integrity of the device and / or delivery system and / or lead to device failure, which may result in patient injury, illness, or death. Reuse, reprocessing, or re-sterilization may also create a risk of contamination of the device and / or cause patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device and / or delivery system may lead to injury, illness, or death of the patient.
- The Esprit™ BTK System is intended to perform as a system. The scaffold should not be removed for use with other dilatation catheters.
- The Esprit™ BTK System should not be used in conjunction with other non-everolimus drug eluting devices in the same vessel as the Esprit™ BTK Scaffold.
- It is not recommended to use this scaffold to treat lesions located at any joint or other hinge points, such as the knee or ankle. The recommended region for below-the-knee (BTK) treatment with the Esprit™ BTK Scaffold is the infrapopliteal arteries at a location ≥ 10 cm above the proximal margin of the ankle mortise. The Esprit™ BTK Scaffold has not been tested for use outside the recommended implant locations.
- This product should not be used in patients with aneurysms immediately adjacent to the scaffold implantation site.
- Insertion of the Esprit™ BTK System and implantation of the scaffold should be performed only under fluoroscopic observation with radiographic equipment providing high resolution images.
- Quantitative imaging is strongly recommended to accurately measure and confirm appropriate vessel sizing (reference vessel diameter ≥ 2.5 mm). If quantitative imaging determines a vessel size < 2.5 mm, do not implant the Esprit™ BTK scaffold.
- Adequate lesion preparation prior to scaffold implantation is required to ensure safe delivery of the scaffold across the target lesion. It is not recommended to treat patients having a lesion that prevents complete inflation of an angioplasty balloon.
- Successful pre-dilatation with residual diameter stenosis of < 30% by visual estimation is required for treatment of the target lesion; < 20% by visual estimation is preferred.
- Ensure the scaffold is not post-dilated beyond the allowable expansion limits.
- Use of appropriate anticoagulant and / or antiplatelet therapy per standard of care is recommended for use of this scaffold system.
- This product should not be used in patients who are not likely to comply with the recommended antiplatelet therapy.
- Judicious selection of patients is necessary, since the use of this device carries the associated risk of scaffold thrombosis, vascular complications, and / or bleeding events.
Precautions
- Scaffold placement should not be performed in patients with known allergies to contrast agent that cannot be medically managed.
- It is not recommended to treat patients having a lesion with excessive tortuosity proximal to or within the lesion.
- When multiple scaffolds are required, only combinations of Esprit™ BTK Scaffolds must be used. Any potential interaction with other drug-eluting or coated devices has not been evaluated.
- The delivery system is intended for deployment of the scaffold only and should not be used to dilate other locations.
- Implantation of the scaffold should be performed only by physicians who have received appropriate training.
- As with all catheter-based procedures, scaffold placement should be performed at facilities where patient can be prepared for necessary intervention and / or surgical removal of the device and vessel repair as per facility protocol.
- Pre-dilatation should be performed with an angioplasty balloon. Cutting or scoring balloons can be used per physician discretion, if the lesion appears to be mildly calcified.
- Failure to pre-dilate the vessel may impair nominal / optimal scaffold delivery.
- Implanting a scaffold may lead to dissection of the vessel distal and / or proximal to the scaffold, requiring additional intervention.
Note: In cases of bailouts, bailout treatment of the target lesion can be done using the Esprit™ BTK Scaffold of the appropriate length. If an appropriate length Esprit™ BTK Scaffold is not available, physicians should use standard of care. - An unexpanded scaffold may be retracted into the introducer sheath one time only. An unexpanded scaffold should not be reintroduced into the artery once it has been pulled back into the introducer sheath.
- Post-dilatation is strongly recommended for optimal scaffold apposition. When performed, post-dilatation should be performed at high pressure (> 16 atm) with a non-compliant balloon up to 0.5 mm larger than the nominal scaffold diameter.
- Use an appropriately sized non-drug coated balloon to pre-dilate the lesion. When treating a long lesion, scaffold the distal portion of the lesion prior to scaffolding the proximal portion of the lesion.
- Ensure that the scaffolded area covers the entire lesion / dissection site and that no gaps exist between scaffolds.
- The extent of the patient’s exposure to drug and polymer is directly related to the number of scaffolds implanted. The safety of everolimus, polymer, and polymer breakdown products was evaluated in pre-clinical studies and the biocompatibility assessment of the Esprit™ BTK Scaffold.
- The safety and effectiveness of the Esprit™ BTK Scaffold in patients with prior brachytherapy of the target lesion or the use of brachytherapy for treated-site restenosis in the Esprit™ BTK Scaffold have not been established. Both vascular brachytherapy and the Esprit™ BTK Scaffold alter arterial modeling. The potential combined effect on arterial remodeling by these two treatments is not known.
- The safety and effectiveness of the Esprit™ BTK System have not been established in clinical trials with the use of either mechanical atherectomy devices (directional atherectomy catheters, rotational atherectomy catheters) or laser atherectomy catheters.
- Formal drug interaction studies have not been performed with the Esprit™ BTK Scaffold because of limited exposure to everolimus eluted from the scaffold.
- Everolimus, the Esprit™ BTK Scaffold’s active pharmaceutical ingredient, is an immunosuppressive agent. Therefore, consideration should be given to patients taking other immunosuppressive agents or who are at risk for immune suppression.
- Oral everolimus use in renal transplant and advanced renal cell carcinoma patients was associated with increased serum cholesterol and triglyceride levels, which in some cases required treatment.
- Non-clinical testing has demonstrated the Esprit™ BTK Scaffold is MR Conditional. A person with the Esprit™ BTK Scaffold may be safely scanned under the following conditions. Failure to follow these conditions may result in injury
- Static magnetic field strength of 7 Tesla or less
- The Esprit™ BTK Scaffold should not migrate in this MRI environment. MRI at 7 Tesla or less may be performed immediately following the implantation of the Esprit™ BTK Scaffold.
Potential Adverse Events
Potential adverse events include, but are not limited to:
Allergic reaction or hypersensitivity to contrast agent, anesthesia, scaffold materials (poly[L-lactide] [PLLA], poly[D, L-lactide] [PDLLA], platinum, or everolimus), and drug reactions to anticoagulation or antiplatelet drugs
- Vascular access complications which may require transfusion or vessel repair, including:
- Catheter site reactions
- Bleeding (ecchymosis, oozing, hematoma, hemorrhage, retroperitoneal hemorrhage)
- Arteriovenous fistula, pseudoaneurysm, aneurysm, dissection, perforation / rupture, and laceration
- Embolism (air, tissue, plaque, thrombotic material, or device)
- Peripheral ischemia
- Target artery complications which may require additional intervention, including:
- Total occlusion or abrupt closure
- Arteriovenous fistula, pseudoaneurysm, aneurysm, dissection, perforation / rupture
- Embolism (air, tissue, plaque, thrombotic material, or device)
- Artery or scaffold thrombosis
- Stenosis or restenosis
- Vasospasm
- Tissue prolapse / plaque shift
- Bleeding (non-access site)
- Additional surgery such as peripheral artery bypass graft surgery or amputation
- Peripheral nerve injury, neuropathy
- Compartment syndrome
- Tissue necrosis, gangrene, ulcer and acute limb ischemia
- Reperfusion injury
- New or worsening pain
- Intervention due to
- Damaged scaffolds
- Partial scaffold deployment
- Scaffold migration / unintentional placement of scaffold
- Other general surgical risks, including:
- Cardiac arrhythmias (including conduction disorders, atrial and ventricular arrhythmias, and blocks)
- Stroke / cerebrovascular accident (CVA) and transient ischemic attack (TIA)
- Venous thromboembolism (including pulmonary embolism)
- Nausea and vomiting
- Hypotension / hypertension
- Infection – local and systemic (including post-procedural)
- Fever
- Blood cell disorders including heparin induced thrombocytopenia (HIT) and other coagulopathy
- Death
- System organ failures:
- Cardiac Failure
- Cardio-respiratory arrest (including pulmonary edema)
- Respiratory failure
- Renal failure
- Shock
The risks described below include the anticipated adverse events referenced in the contraindications, warnings, and precautions sections of the everolimus labels / SmPCs and / or observed at incidences ≥ 10% in clinical trials with oral everolimus for different indications. Refer to the drug SmPCs and labels for more detailed information and less frequent adverse events.
- Abdominal pain
- Anemia
- Angioedema (increased risk with concomitant angiotensin converting enzyme [ACE] inhibitor use)
- Arterial thrombotic events
- Bleeding and coagulopathy (including hemolytic uremic syndrome [HUS], thrombotic thrombocytopenic purpura [TTP], and thrombotic microangiopathy; increased risk with concomitant cyclosporine use)
- Constipation
- Cough
- Diabetes mellitus
- Diarrhea
- Dyspnea
- Embryo-fetal toxicity
- Erythema
- Erythroderma
- Headache
- Hepatic artery thrombosis (HAT)
- Hepatic disorders (including hepatitis and jaundice)
- Hypersensitivity to everolimus active substance, or to other rapamycin derivates
- Hypertension
- Infections (bacterial, viral, fungal, or protozoan infections, including infections with opportunistic pathogens). Polyoma virus-associated nephropathy (PVAN), JC virus associated progressive multiple leukoencephalopathy (PML), fatal infections and sepsis have been reported in patients treated with oral everolimus.
- Kidney arterial and venous thrombosis
- Laboratory test alterations (elevations of serum creatinine, proteinuria, hypokalemia, hyperkalemia; hyperglycemia, dyslipidemia including hypercholesterolemia and hypertriglyceridemia; abnormal liver function tests; decreases in hemoglobin, lymphocytes, neutrophils, and platelets)
- Lymphoma and skin cancer
- Male infertility
- Menstrual irregularities
- Nausea
- Nephrotoxicity (in combination with cyclosporine)
- Non-infectious pneumonitis (including interstitial lung disease)
- Oral ulcerations
- Pain
- Pancreatitis
- Pericardial effusion
- Peripheral edema
- Pleural effusion
- Pneumonia
- Pyrexia
- Rash
- Renal failure
- Upper respiratory tract infection
- Urinary tract infection
- Venous thromboembolism
- Vomiting
- Wound healing complications (including wound infections and lymphocele)
There may be other potential adverse events that are unforeseen at this time.
MAT-2403616 v1.0
SurVeil‡ Drug-Coated
Balloon (DCB)

Indications
The SurVeil‡ DCB is indicated for percutaneous transluminal angioplasty, after appropriate vessel preparation, of de novo or restenotic lesions (≤ 180 mm in length) in femoral and popliteal arteries having reference vessel diameters of 4 mm to 7 mm.
Contraindications
The SurVeil‡ DCB is contraindicated for use in:
- Patients who cannot receive recommended antiplatelet and/or anticoagulant therapy
- Patients with known hypersensitivity to paclitaxel or structurally related compounds
- Patients judged to have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the delivery system
- Women who are breastfeeding, pregnant or are intending to become pregnant or men intending to father children
- Coronary, renal and supra-aortic/cerebrovascular arteries
Warnings
- Adhere to the following use parameters for the index procedure:
- Do not use more balloons than necessary. No more than 200 mm of total balloon length should be used, for a total maximum treatable length of 180 mm.
- This product should not be used bilaterally or in multiple lesions that cannot be treated with up to 200 mm total balloon length.
- The safety of exposure to higher doses of the paclitaxel/polyethyleneimine (PEI) drug coating has not been established.
- The SurVeil‡ DCB is supplied STERILE for SINGLE USE ONLY. Do not reuse and/or resterilize.
- Do not open sterile package until you are ready to begin the procedure.
- Do not use if the integrity of the sterile package has been compromised or if any sterile package or product defects are noted.
- Do not use after the Use by Date on the label.
- Do not exceed the rated burst pressure (RBP) recommended in the compliance chart for this device specified on device packaging.
- To minimize the potential for vessel damage, ensure the expected inflated diameter of the balloon approximates the intended treatment segment.
- Do not use any gaseous medium to inflate the balloon.
- Do not use device if air does not aspirate properly.
- Completely deflate the balloon and maintain negative pressure before withdrawing it from the dilated area.
- The safety and effectiveness of utilizing multiple SurVeil‡ DCBs with a total drug dosage exceeding 9048 μg of paclitaxel in a patient has not been clinically evaluated in the TRANSCEND trial.
DO NOT REUSE and/or RESTERILIZE the SurVeil‡ DCB. Reuse and/or resterilization may create a risk of contamination of the device and/or cause patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s). Contamination of the device may lead to injury, illness, or death of the patient. Reuse and/or resterilization may compromise integrity of the device, including the drug coating, or lead to device failure, which may result in patient injury, illness, or death. Surmodics is not responsible for any direct, incidental, or consequential damages resulting from reuse and/or resterilization.
Precautions
- The SurVeil‡ DCB is only to be used by clinicians trained in peripheral vascular percutaneous interventional procedures. A thorough understanding of the technical principles, clinical applications and risks associated with percutaneous transluminal angioplasty is necessary before using the SurVeil‡ DCB.
- Consideration should be given to the risks and benefits of use in patients with a history of non-controllable allergies to contrast solution.
- Use only the recommended balloon inflation solution (50% contrast / 50% sterile saline).
- Administer appropriate drug therapy to the patient according to standard protocols for PTA before insertion of the dilatation catheter.
- Take precautions to prevent or reduce clotting when any catheter is used. Flush and rinse all products entering the vascular system with heparinized normal saline or a similar solution. For the SurVeil‡ DCB, flush the guidewire lumen through the guidewire port with heparinized normal saline until the fluid exits the distal tip. Do not rinse or wipe the SurVeil‡ DCB.
- Keep the SurVeil‡ DCB dry prior to insertion into the body. Replace any device that has come into contact with fluids prior to use.
- Do not immerse the SurVeil‡ DCB in a saline bath.
- Handle the SurVeil‡ DCB only with dry sterile gloves.
- Avoid moisture contact with the balloon.
- Minimize contact with the coated balloon. Extended manipulation of the SurVeil‡ DCB can cause loss of coating integrity.
- Keep the balloon sheath in place during preparation of the SurVeil‡ DCB. Remove the balloon sheath immediately before placing over guidewire.
- If difficulty is encountered while removing the balloon sheath, discard device and use a new SurVeil‡ DCB.
- Do not attempt to pass the SurVeil‡ DCB through an introducer that is smaller than indicated in the list of required materials or on the primary package label.
- Do not inflate the balloon outside the body or prior to reaching the target segment as it may disrupt the drug coating.
- Always advance and withdraw the SurVeil‡ DCB under negative pressure.
- Do not use the SurVeil‡ DCB if the shaft has been bent or kinked because device function could be compromised.
- Do not advance the SurVeil‡ DCB if resistance is met.
- Do not move the guidewire or reposition once inflation has begun.
- Only change the position of the balloon catheter with the guidewire in place.
- Do not over-tighten the hemostatic valve around the SurVeil‡ DCB as lumen constriction may occur, affecting inflation/deflation of the balloon. Advance the SurVeil‡ DCB to the target segment in an efficient manner and immediately inflate.
- To prevent over-pressurization, use a pressure monitoring device.
- Minimize the number of contrast solution injections during positioning to ensure appropriate drug delivery to lesion.
- Use of the SurVeil‡ DCB in conjunction with other drug-eluting stents or drug-coated balloons in the same procedure or following treatment failure has not been evaluated.
Pre- and post-procedure medication regimen
It is strongly advised that the treating physician follow the Inter- Society Consensus (TASC II) Guidelines recommendations (or other applicable country guidelines) for antiplatelet therapy pre- and post-procedure
Potential Adverse Events
Potential adverse events, which may be associated with the use of a peripheral-dilatation balloon catheter procedure may include, but are not limited to, the following:
- Acute re-occlusion necessitating surgical intervention
- Allergic reaction to contrast solution, anti-platelet therapy, or catheter system components
- Amputation
- Aneurysm
- Arrhythmias
- Arterio-venous fistula
- Bleeding
- Death
- Endocarditis
- Femoral nerve compression with associated neuropathy
- Groin area bruising and discomfort
- Ischemia or infarction of tissue/organ
- Renal insufficiency or failure
- Local hematoma
- Local hemorrhage
- Local infections
- Local or distal thromboembolic episodes
- Low blood pressure
- Pain and tenderness
- Pseudoaneurysm
- Pyrogenic reaction
- Respiratory failure
- Restenosis of the dilated artery
- Sepsis/infection
- Short-term hemodynamic deterioration
- Stroke
- Systemic embolization
- Total occlusion or thrombosis
- Vessel damage, dissection, perforation, rupture, or spasm
Potential adverse events that may be unique to the paclitaxel drug coating may include, but are not limited to:
- Allergic/immunologic reaction
- Alopecia
- Anemia
- Gastrointestinal symptoms
- Hematologic dyscrasia (including leukopenia, neutropenia, thrombocytopenia)
- Hepatic enzyme changes
- Histologic changes in vessel wall, including inflammation, cellular damage, or necrosis
- Myalgia/arthralgia
- Myelosuppression
- Peripheral neuropathy
SurVeil‡ is manufactured by Surmodics, Inc., distributed by Abbott Group of Companies
MAT-2213996 v1.0
Supera™ Peripheral Stent System

Indications
The Supera™ Peripheral Stent System is indicated to improve luminal diameter in the treatment of patients with symptomatic de novo or restenotic native lesions or occlusions of the superficial femoral artery (SFA) and / or proximal popliteal artery with reference vessel diameters of 4.0 to 7.5 mm, and lesion lengths up to 140 mm.
Contraindications
The Supera™ Peripheral Stent System is contraindicated in:
- Patients who are judged to have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the stent or stent delivery system.
- Patients who cannot receive antiplatelet or anticoagulation therapy. Based on in vivo thrombogenicity testing, the device should not be used in patients who cannot be anticoagulated as there may be some thrombus formation in the absence of anticoagulation.
Warnings
- This device is intended for single-use only. Do not reuse. Do not resterilize. Do not use if the package is opened or damaged.
- Use this device prior to the “Use By” date as specified on the device package label. Store in a dry, dark, cool place.
- DO NOT use if it is suspected that the sterility of the device has been compromised.
- Persons with known hypersensitivities to Nitinol and / or its components (e.g. nickel-titanium) may suffer an allergic reaction to this implant.
- Administer appropriate antiplatelet therapy pre- and post-procedure.
- Careful attention should be paid when sizing and deploying the stent to prevent stent elongation. In the SUPERB clinical study, stent elongation was associated with a decrease in patency at 12 months.
Precautions
The Supera™ Peripheral Stent System should only be used by physicians and medical personnel trained in vascular interventional techniques and trained on the use of this device.
- The long-term safety and effectiveness of the Supera™ Peripheral Stent System has not been established beyond three years.
- The safety and effectiveness of the Supera™ Peripheral Stent System has not been established in patients who:
- are less than 18 years old
- are pregnant or lactating
- have in-stent restenosis of the target lesion
- have known hypersensitivity to any component of the stent system (e.g., nickel)
- cannot tolerate contrast media and cannot be pre-treated
- have uncontrolled hypercoagulability and / or another coagulopathy
- This device is not designed for use with contrast media injection systems or power injection systems.
- The flexible design of the Supera™ stent may result in variation in the deployed stent length.
Magnetic Resonance Imaging (MRI) Safety Information
Nonclinical testing has demonstrated that the Supera™ stent, in single and in overlapped configurations up to 250 mm in length, is MR Conditional. A patient with this device can be safely scanned in an MR system meeting the following conditions:
- Static magnetic field of 1.5 or 3.0 Tesla
- Maximum spatial gradient magnetic field of 2,500 Gauss/cm (25 T/m)
- Maximum MR whole-body-averaged specific absorption rate (SAR) of
- 2 W/kg for landmarks (i.e. center of RF coil) above the umbilicus
- 1 W/kg for landmarks below the umbilicus and above the mid-thigh
- 0.5 W/kg for landmarks below the mid-thigh
Under the scan conditions defined above, the Supera™ stent is expected to produce a maximum temperature rise of 7.6 °C after 15 minutes of continuous scanning.
In nonclinical testing, the image artifact caused by the device extends approximately 2 cm from the Supera™ stent when imaged with a gradient echo or spin echo sequence and a 3T MRI system.
Potential Adverse Events
Potential adverse events include, but are not limited to:
- Abrupt closure
- Allergic reaction (contrast medium; drug; stent material)
- Amputation or limb loss
- Aneurysm or pseudoaneurysm in vessel or at vascular access site
- Angina or coronary ischemia
- Arrhythmia (including premature beats, bradycardia, atrial or ventricular tachycardia, atrial or ventricular fibrillation)
- Arteriovenous fistula
- Bleeding complications requiring transfusion or surgical intervention
- Death
- Detachment of a system component or implantation in an unintended site
- Embolization, arterial or other (e.g. air, tissue, plaque, thrombotic material, or stent)
- Emergent surgery
- Fever
- Hematoma or hemorrhagic event, with or without surgical repair
- Hyperperfusion syndrome
- Hypertension / Hypotension
- Infection
- Myocardial infarction
- Pain (leg, foot, and/or insertion site)
- Partial stent deployment
- Peripheral nerve injury
- Pulmonary embolism
- Renal failure or insufficiency
- Restenosis of vessel in stented segment
- Shock
- Stent malapposition or migration, which may require emergency surgery to remove stent
- Stent strut fracture
- Thrombosis or occlusion
- Stroke
- Transient ischemic attack
- Venous thromboembolism
- Vessel dissection, perforation or rupture
- Vessel spasm or recoil
- Worsening claudication or rest pain
MAT-2103597 v3.0
Perclose™ ProStyle™ Suture-Mediated Closure and Repair (SMCR) System

Indications:
The Perclose™ ProStyle™ Suture-Mediated Closure and Repair System is indicated for the percutaneous delivery of suture for closing the common femoral artery and vein access sites of patients who have undergone diagnostic or interventional catheterization procedures.
The Perclose™ ProStyle™ SMCR System is indicated for closing the common femoral vein in single or multiple access sites per limb.
The Perclose™ ProStyle™ SMCR System is used without or, if required, with adjunctive manual compression.
For access sites in the common femoral artery using 5F to 21F sheaths. For arterial sheath sizes greater than 8F, at least two devices and the pre-close technique are required.
For access sites in the common femoral vein using 5F to 24F sheaths. For venous sheath sizes greater than 14F, at least two devices and the pre-close technique are required.
Caution:
Federal law restricts this medical device to sale by or on the order of a physician (or allied healthcare professionals, authorized by, or under the direction of, such physicians) who is trained in diagnostic and / or interventional catheterization procedures and who has been trained by an authorized representative of Abbott.
Prior to use, the operator must review the Instructions for Use and be familiar with the deployment techniques associated with the use of this device.
During closure of access sites using a procedural sheath greater than 8F, it is recommended that a vascular surgeon or a surgeon with vascular training be available in case surgical conversion to control bleeding and to repair the vessel is needed.
Contraindications:
There are no known contraindications to the use of this device.
Warnings:
Do not use the Perclose™ ProStyle™ SMCR System if the packaging or sterile barrier has been previously opened or damaged or if the components appear to be damaged or defective.
DO NOT RESTERILIZE OR REUSE. The Perclose™ ProStyle™ SMCR System is intended for single use only.
Do not use the Perclose™ ProStyle™ SMCR System if the sterile field has been broken where bacterial contamination of the sheath or surrounding tissues may have occurred, since such a broken sterile field may result in infection.
Do not use the Perclose™ ProStyle™ SMCR System if the puncture site is located above the most inferior border of the inferior epigastric artery (IEA) and / or above the inguinal ligament based upon bony landmarks, since such a puncture site may result in a retroperitoneal hematoma. Perform a femoral angiogram to verify the location of the puncture site. Note: This may require both a right anterior oblique (RAO) and left anterior oblique (LAO) angiogram to adequately visualize where the sheath enters the femoral vessel.
Do not use the Perclose™ ProStyle™ SMCR System in arterial or venous access if the puncture is through the posterior wall or if there are multiple punctures in the same access site, since such punctures may result in a hematoma or retroperitoneal bleed.
Do not use the Perclose™ ProStyle™ SMCR System if the puncture site is located in the superficial femoral artery or the profunda femoris artery, or the bifurcation of these vessels, since such puncture sites may result in a pseudoaneurysm, intimal dissection, or an acute vessel closure (thrombosis of small artery lumen). Perform a femoral angiogram to verify the location of the puncture site. Note: This may require both a right anterior oblique (RAO) and left anterior oblique (LAO) angiogram to adequately visualize where the sheath enters the femoral vessel.
Precautions:
- Prior to use, inspect the Perclose™ ProStyle™ SMCR System to ensure that the sterile packaging has not been damaged during shipment. Examine all components prior to use to verify proper function. Exercise care during device handling to reduce the possibility of accidental device breakage.
- As with all catheter-based procedures, infection is a possibility. Observe sterile technique at all times when using the Perclose™ ProStyle™ SMCR System. Employ appropriate groin management, as per hospital protocol, post-procedure, and post-hospital discharge to prevent infection.
- Use a single wall puncture technique. Do not puncture the posterior wall of the vessel in arterial and venous access.
- Do not deploy the Perclose™ ProStyle™ Device at an elevated angle against resistance as this may cause a cuff miss or device breakage.
- There are no reaccess restrictions if previous arteriotomy / venotomy repairs were achieved with Abbott Medical SMC or SMCR systems.
- If significant blood flow is present around the Perclose™ ProStyle™ Device, do not deploy needles. Remove the device over a 0.038" (0.97 mm) (or smaller) guide wire and insert an appropriately sized sheath.
- Prior to depressing the plunger to advance the needles, stabilize the device by the body to ensure the foot is apposed to the vessel wall and the device does not twist during deployment. Twisting (torquing) of the device could lead to needle deflection resulting in a cuff miss. Do not use excessive force or repeatedly depress the plunger. Excessive force on the plunger during deployment could potentially cause breakage of the device, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- Do not apply excessive force to the lever when opening the foot and returning the foot to its original position down to the body of the device. Do not attempt to remove the device without closing the lever. Excessive force on the lever or attempting to remove the device without closing the lever could cause breakage of the device and / or lead to vessel trauma, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- Do not advance or withdraw the Perclose™ ProStyle™ Device against resistance until the cause of that resistance has been determined. Excessive force used to advance or torque the Perclose™ ProStyle™ Device should be avoided, as this may lead to significant vessel damage and / or breakage of the device, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- If excessive resistance in advancing the Perclose™ ProStyle™ Device is encountered, withdraw the device over a 0.038" (0.97 mm) (or smaller) guide wire and reinsert the introducer sheath or use manual compression.
- Remove the Perclose™ ProStyle™ sheath before tightening the suture. Failure to remove the sheath prior to tightening the suture may result in detachment of the tip of the sheath.
- Care should be taken to avoid damage to the suture from handling. Avoid crushing damage due to application of surgical instruments such as clamps, forceps or needle holders.
- For catheterization procedures using a 5F – 8F procedural sheath, use manual compression in the event that bleeding from the femoral access site persists after the use of the Perclose™ ProStyle™ SMCR System to obtain hemostasis.
- For catheterization procedures using a procedural sheath > 8F, use manual compression, compression assisted devices, surgical repair, and / or other appropriate treatment methods in the event that bleeding from the femoral access site persists after the use of the Perclose™ ProStyle™ SMCR System to obtain hemostasis.
- For catheterization procedures using a procedural sheath > 8F, where the operating physician is not a vascular surgeon, it is recommended that a vascular surgeon or a surgeon with vascular training be available during the procedure to perform any necessary vascular surgical intervention.
- If the Perclose™ ProStyle™ Device is used to close and repair multiple access sites in the same vessel, space the access sites apart adequately to minimize sheath-device interference.
Potential Adverse Events:
Potential adverse events associated with use of vessel closure devices may include, but are not limited to, the following:
- Allergic reaction or hypersensitivity to device components
- Vascular access complications which may require transfusion or vessel repair, including:
- Anemia
- Aneurysm
- Arteriovenous fistula
- Bleeding / hemorrhage / re-bleeding
- Bruising
- Hematoma
- Embolism
- Inflammation
- Intimal tear / dissection
- Perforation
- Pseudoaneurysm
- Retroperitoneal hematoma / bleeding
- Scar formation
- Wound dehiscence
- Cardiac arrhythmias (including conduction disorders, atrial and ventricular arrhythmias)
- Atrial arrhythmias
- Ventricular arrhythmias
- Femoral artery / venous complications which may require additional intervention, including:
- Arterial / venous stenosis
- Arterial / venous occlusion
- Arteriovenous fistula
- Intimal tear / dissection
- Ischemia distal to closure site
- Nerve injury
- Numbness
- Thrombus formation
- Vascular injury
- Venous thromboembolism (including deep vein thrombosis, pulmonary embolism, post-procedure pulmonary embolism)
- Infection - local or systemic
- Pain
- Hemodynamic instability:
- Hypotension / hypertension
- Vasovagal episode
- Death
- Device complications
- Device failure
- Device malfunction
MAT-2100368 v4.0
Perclose ProGlide™ Suture-Mediated Closure (SMC) System

Indications:
The Perclose ProGlide™ Suture-Mediated Closure System is indicated for the percutaneous delivery of suture for closing the common femoral artery and vein access site of patients who have undergone diagnostic or interventional catheterization procedures.
The Perclose ProGlide™ SMC System is indicated for closing the common femoral vein in single or multiple access sites per limb.
The Perclose ProGlide™ SMC System is used without or, if required, with adjunctive manual compression.
For access sites in the common femoral artery using 5F to 21F sheaths. For arterial sheath sizes greater than 8F, at least two devices and the pre-close technique are required.
For access sites in the common femoral vein using 5F to 24F sheaths. For venous sheath sizes greater than 14F, at least two devices and the pre-close technique are required.
Caution:
Federal law restricts this medical device to sale by or on the order of a physician (or allied healthcare professionals, authorized by, or under the direction of, such physicians) who is trained in diagnostic and / or interventional catheterization procedures and who has been trained by an authorized representative of Abbott.
Prior to use, the operator must review the Instructions for Use and be familiar with the deployment techniques associated with the use of this device.
During closure of access sites using a procedural sheath greater than 8F, it is recommended that a vascular surgeon or a surgeon with vascular training be available in case surgical conversion to control bleeding and to repair the vessel is needed.
Contraindications:
There are no known contraindications to the use of this device.
Warnings:
Do not use the Perclose ProGlide™ SMC System if the packaging or sterile barrier has been previously opened or damaged or if the components appear to be damaged or defective.
DO NOT RESTERILIZE OR REUSE. The Perclose ProGlide™ SMC System is intended for single use only.
Do not use the Perclose ProGlide™ SMC System if the sterile field has been broken where bacterial contamination of the sheath or surrounding tissues may have occurred, since such a broken sterile field may result in infection.
Do not use the Perclose ProGlide™ SMC System if the puncture site is located above the most inferior border of the inferior epigastric artery (IEA) and / or above the inguinal ligament based upon bony landmarks, since such a puncture site may result in a retroperitoneal hematoma. Perform a femoral angiogram to verify the location of the puncture site. Note: This may require both a right anterior oblique (RAO) and left anterior oblique (LAO) angiogram to adequately visualize where the sheath enters the femoral vessel.
Do not use the Perclose ProGlide™ SMC System in arterial or venous access if the puncture is through the posterior wall or if there are multiple punctures in the same access site, since such punctures may result in a hematoma or retroperitoneal bleed.
Do not use the Perclose ProGlide™ SMC System if the puncture site is located in the superficial femoral artery or the profunda femoris artery, or the bifurcation of these vessels, since such puncture sites may result in a pseudoaneurysm, intimal dissection, or an acute vessel closure (thrombosis of small artery lumen). Perform a femoral angiogram to verify the location of the puncture site. Note: This may require both a right anterior oblique (RAO) and left anterior oblique (LAO) angiogram to adequately visualize where the sheath enters the femoral vessel.
Precautions:
- Prior to use, inspect the Perclose ProGlide™ SMC System to ensure that the sterile packaging has not been damaged during shipment. Examine all components prior to use to verify proper function. Exercise care during device handling to reduce the possibility of accidental device breakage.
- As with all catheter-based procedures, infection is a possibility. Observe sterile technique at all times when using the Perclose ProGlide™ SMC System. Employ appropriate groin management, as per hospital protocol, post procedure and post hospital discharge to prevent infection.
- Use a single wall puncture technique. Do not puncture the posterior wall of the vessel in arterial and venous access.
- Do not deploy the Perclose ProGlide™ Device at an elevated angle against resistance as this may cause a cuff miss or device breakage.
- There are no reaccess restrictions if previous arteriotomy / venotomy repairs were achieved with Abbott Medical SMC or SMCR systems.
- If significant blood flow is present around the Perclose ProGlide™ Device, do not deploy needles. Remove the device over a 0.038" (0.97 mm) (or smaller) guide wire and insert an appropriately sized sheath.
- Prior to depressing the plunger to advance the needles, stabilize the device by the body to ensure the foot is apposed to the vessel wall and the device does not twist during deployment. Twisting (torquing) of the device could lead to needle deflection resulting in a cuff miss. Do not use excessive force or repeatedly depress the plunger. Excessive force on the plunger during deployment could potentially cause breakage of the device, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- Do not apply excessive force to the lever when opening the foot and returning the foot to its original position down to the body of the device. Do not attempt to remove the device without closing the lever. Excessive force on the lever or attempting to remove the device without closing the lever could cause breakage of the device and / or lead to vessel trauma, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- Do not advance or withdraw the Perclose ProGlide™ Device against resistance until the cause of that resistance has been determined. Excessive force used to advance or torque the Perclose ProGlide™ Device should be avoided, as this may lead to significant vessel damage and / or breakage of the device, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- If excessive resistance in advancing the Perclose ProGlide™ Device is encountered, withdraw the device over a 0.038" (0.97 mm) (or smaller) guide wire and reinsert the introducer sheath or use manual compression.
- Remove the Perclose ProGlide™ sheath before tightening the suture. Failure to remove the sheath prior to tightening the suture may result in detachment of the tip of the sheath.
- Care should be taken to avoid damage to the suture from handling. Avoid crushing damage due to application of surgical instruments such as clamps, forceps or needle holders.
- For catheterization procedures using a 5F – 8F procedural sheath, use manual compression in the event that bleeding from the femoral access site persists after the use of the Perclose ProGlide™ SMC System to obtain hemostasis.
- For catheterization procedures using a procedural sheath > 8F, use manual compression, compression assisted devices, surgical repair, and / or other appropriate treatment methods in the event that bleeding from the femoral access site persists after the use of the Perclose ProGlide™ SMC System to obtain hemostasis.
- For catheterization procedures using a procedural sheath > 8F, where the operating physician is not a vascular surgeon, it is recommended that a vascular surgeon or a surgeon with vascular training be available during the procedure to perform any necessary vascular surgical intervention.
- If the Perclose ProGlide™ Device is used to close and repair multiple access sites in the same vessel, space the access sites apart adequately to minimize sheath-device interference
Potential Adverse Events:
Potential adverse events associated with use of vessel closure devices may include, but are not limited to, the following:
- Allergic reaction or hypersensitivity to device components
- Vascular access complications which may require transfusion or vessel repair, including:
- Anemia
- Aneurysm
- Arteriovenous fistula
- Bleeding / hemorrhage / re-bleeding
- Bruising
- Hematoma
- Embolism
- Inflammation
- Intimal tear / dissection
- Perforation
- Pseudoaneurysm
- Retroperitoneal hematoma / bleeding
- Scar formation
- Wound dehiscence
- Cardiac arrhythmias (including conduction disorders, atrial and ventricular arrhythmias)
- Atrial arrhythmias
- Ventricular arrhythmias
- Femoral artery / venous complications which may require additional intervention, including:
- Arterial / venous stenosis
- Arterial / venous occlusion
- Arteriovenous fistula
- Intimal tear / dissection
- Ischemia distal to closure site
- Nerve injury
- Numbness
- Thrombus formation
- Vascular injury
- Venous thromboembolism (including deep vein thrombosis, pulmonary embolism, post-procedure pulmonary embolism)
- Infection – local or systemic
- Pain
- Hemodynamic instability:
- Hypotension / hypertension
- Vasovagal episode
- Death
- Device complications
- Device failure
- Device malfunction
MAT-2100358 v4.0
StarClose SE™ Vascular Closure System

Indications for Use
The StarClose SE™ Vascular Closure System is indicated for the percutaneous closure of common femoral artery access sites while reducing times to hemostasis, ambulation, and dischargeability in patients who have undergone diagnostic endovascular catheterization procedures utilizing a 5F or 6F procedural sheath.
The StarClose SE™ Vascular Closure System is indicated for use to allow patients who have undergone diagnostic endovascular catheterization procedures to ambulate and be eligible for discharge as soon as possible after device placement.
The StarClose SE™ Vascular Closure System is indicated for the percutaneous closure of common femoral artery access sites while reducing times to hemostasis and ambulation in patients who have undergone interventional endovascular catheterization procedures utilizing a 5F or 6F procedural sheath.
Caution
Federal law restricts this device to sale by or on the order of a physician (or allied healthcare professionals, authorized by, or under the direction of, such physicians) who is trained in diagnostic and therapeutic catheterization procedures and who has been trained by an authorized representative of Abbott Vascular.
Prior to use, the operators must review the Instructions for Use and be familiar with the deployment techniques associated with the use of this device.
Contraindications
The StarClose SE™ Vascular Closure System is contraindicated for use in patients with known hypersensitivity to nickel-titanium.
Warnings
Do not use the StarClose SE™ Vascular Closure System if the packaging or sterile barrier has been previously opened or damaged or if the components appear to be damaged or defective.
DO NOT RESTERILIZE OR REUSE. The StarClose SE™ Vascular Closure System and accessories are intended for single use only.
Do not use the StarClose SE™ Vascular Closure System if the sterile field has been broken where bacterial contamination of the sheath or surrounding tissues may have occurred, since such a broken sterile field may result in infection.
Do not use the StarClose SE™ Vascular Closure System if the puncture site is located above the most inferior border of the inferior epigastric artery (IEA) and / or above the inguinal ligament based upon bony landmarks, since such a puncture site may result in a retroperitoneal hematoma. Perform a femoral angiogram to verify the location of the puncture site.
Do not use the StarClose SE™ Vascular Closure System if the puncture is through the posterior wall or if there are multiple punctures, since such punctures may result in a retroperitoneal hematoma.
Do not use the StarClose SE™ Vascular Closure System if the puncture site is located in the superficial femoral artery or the profunda femoris artery, since such puncture sites may result in a pseudoaneurysm, intimal dissection, or an acute vessel closure (thrombosis of small artery lumen). Perform a femoral angiogram to verify the location of the puncture site.
Precautions
- The StarClose SE™ Vascular Closure System should be used only by operators trained in diagnostic and interventional catheterization procedures who have been certified by an authorized representative of Abbott Vascular Inc.
- The StarClose SE™ Vascular Closure System is provided sterile and non-pyrogenic in unopened undamaged packaging. Products are sterilized with ethylene oxide and intended for single use only. Do not resterilize. Store in a cool, dry place.
- Prior to use, inspect the StarClose SE™ Vascular Closure System to ensure that the sterile packaging has not been damaged during shipment. Examine all components prior to use to verify proper function. Exercise care during device handling to reduce the possibility of accidental device breakage.
- As with all catheter-based procedures, infection is a possibility. Observe sterile technique at all times when using the StarClose SE™ Vascular Closure System. Employ appropriate groin management, as per hospital protocol, post-procedure and post-hospital discharge to prevent infection.
- Use a single wall puncture technique. Do not puncture the posterior wall of the artery.
- Do not use the StarClose SE™ Vascular Closure System to close vessels with diameters less than 5 mm.
- Do not deploy the Clip in areas of calcified plaque.
- The StarClose SE™ Vascular Closure System can be used ONLY with the StarClose Exchange System (included in the StarClose SE™ Vascular Closure System packaging).
- Do not advance or withdraw the StarClose SE™ Vascular Closure Device against resistance until the cause of that resistance has been determined. Excessive force used to advance or torque the StarClose SE™ device should be avoided, as this may lead to significant vessel damage and/or breakage of the device, which may necessitate interventional and/or surgical removal of the device and vessel repair.
MRI Safety Information
The StarClose Clip has been shown to be MR Conditional immediately following implantation. A patient with this implant can be scanned safely immediately after clip placement under the following conditions:
- Static magnetic field of 3 Tesla or less
- Spatial gradient magnetic field of 720 Gauss/cm or less
- Maximum MR system reported whole-body-averaged specific absorption rate (SAR) of 3 W/kg for 15 minutes of scanning.
In non-clinical testing, the StarClose Clip produced a temperature rise of 0.5°C at maximum MR system-reported whole-body-averaged specific absorption rate (SAR) of 3 W/kg for 15 minutes of MR scanning in a 3 Tesla MR system using a transmit/receive body coil.
The MR image quality may be compromised if the area of interest is in the exact same area or relatively close to the position of the StarClose Clip. Therefore, optimization of MR imaging parameters to compensate for the presence of this implant may be necessary.
Adverse Events
Potential adverse events that could be associated with the use of this device include:
- Major Vascular Complications (Composite)
- Vascular Injury Requiring Repair
- Surgery
- Angioplasty
- Ultrasound Guided Compression
- Thrombin Injection or Other Percutaneous Procedure
- New Ipsilateral Lower Extremity Ischemia
- Access Site-related Bleeding Requiring Transfusion
- Access Site-related Infection Requiring Intravenous Antibiotics or Prolonged Hospitalization
- Access Site-related Nerve Injury Requiring Intervention
- Death
- Minor Vascular Complications (Composite)
- Pseudoaneurysm
- Arteriovenous Fistula
- Hematoma (≥ 6 cm)
- Late Access Site-related Bleeding
- Transient Lower Extremity Ischemia
- Ipsilateral Deep Vein Thrombosis
- Transient Access Site-related Nerve Injury
- Access Site-related Vessel Injury
- Access Site Wound-related Dehiscence
- Access Site-related Bleeding Requiring ≥ 30 minutes to Re-achieve Hemostasis
- Localized Access Site Infection Treated with IM or Oral Antibiotics
- UADE
MAT-2114590 v3.0