Which Perclose Device Are You Using?

Experience the Difference of Perclose™ ProStyle™
Don't Just Close it. Perclose™ it.
The Perclose™ ProStyle™ Suture-Mediated Closure and Repair (SMCR) System is the next-generation design of the proven and trusted Perclose ProGlide™ SMC System. Each device delivers a pretied suture to the femoral access site to:
- Enable immediate and durable hemostasis1
- Reduce a patient's time to ambulation and discharge2,3
- Lower access site-related complications2,3,4
Key upgrades in the Perclose™ ProStyle™ SMCR System include:
- Higher tensile-strength needles for more reliability in challenging anatomy5
- Additional hydrophilic coating for smoother advancement5
- Enhanced visual cues5
Perclose™ Family of products offers a COMPREHENSIVE, VERSATILE, and PROVEN solution for hemostasis management, unrivaled by any other single offering.
Comprehensive
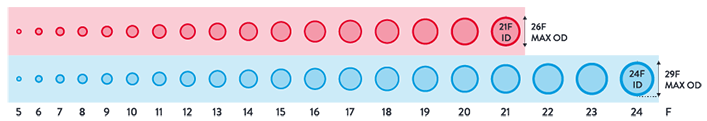
Breadth of Transfemoral Applications
- Same broadest arterial and venous indication among all vascular closure devices on the market*
- Arterial Sheaths 5-21F1,5
(Max OD 26F)7 - Venous Sheaths 5-24F1,6
(Max OD 29F)7
Versatile
Keep Your Options Open
Before the Procedure
Customize your closing approach by deploying the sutures before the procedure (pre-close) or after the procedure (post-close), by using one or more devices per access site1
During the Procedure
Maintain guide wire access even after deployment of the Perclose™ sutures to keep all therapeutic options open.1
After the Procedure
Pull the sheath sooner due to no ACT-level requirements, and re-puncture the same access site immediately or in the near-term since Perclose™ Devices have no reaccess restrictions.1
Proven
Established Decades-Long Track Record
- Robust Clinical Evidence on the safety and efficacy of both venous and arterial access site closure.2,3,4
- 20,000,000+ closures and counting across 100+ countries worldwide8,9
- World's Leading Vascular Closure Device†
Learn how Perclose™ Devices can significantly improve EP lab workflows and enhance the patient experience.
Primary Intention healing through tissue approximation10
Suture-Mediated Closure
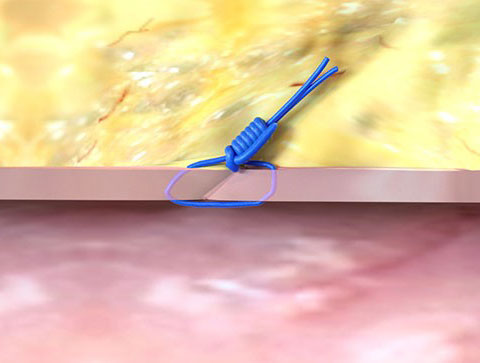
Access the Vessel
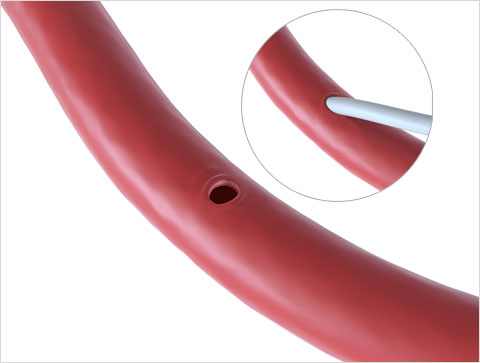
Close the Access Site
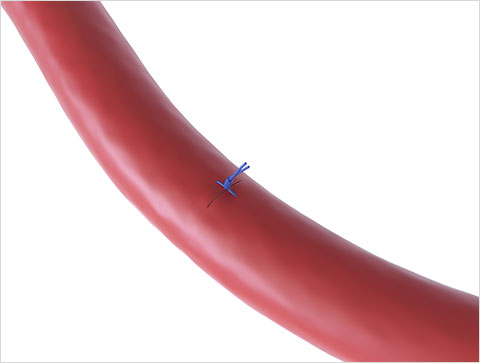
Heal the Vessel
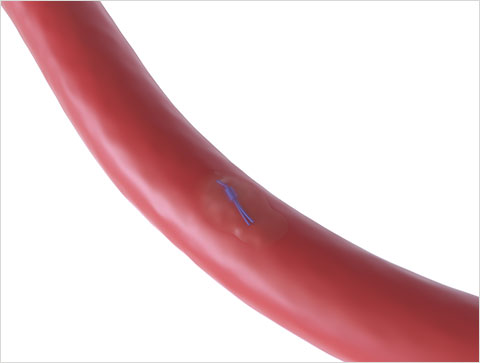
Perclose™ Knot Animation
Frequently Asked Questions
Are the indications the same between Perclose™ ProStyle™ and Perclose ProGlide™?
Yes, the indications are identical between Perclose™ ProStyle™ and Perclose ProGlide™ and each device can be used after the same types of percutaneous transfemoral procedures. These Perclose™ devices still maintain the broadest indication* amongst vessel closure devices in the market:
- Arterial Sheaths 5-21F1,6 (Max OD 26F)8
- Venous Sheaths 5-24F1,7 (Max OD 29F)8
Will Perclose ProGlide™ be discontinued?
The Perclose™ ProStyle™ SMCR System is the next-generation design evolution of the proven and trusted Perclose ProGlide™ SMC System, and it has been improved based on years of customer input and feedback. Although the conversion will take a few years, the updated Perclose™ ProStyle™ SMCR System is an overall better device and we should want our customers to make the switch for improved reliability, enhanced usability, and a more intuitive deployment experience.
What features of Perclose™ ProStyle™ have been updated to improve the reliability and performance over that of Perclose ProGlide™?
Stronger Needles in Perclose™ ProStyle™5
- Designed to reduce the frequency of needle deflection in more complex challenging anatomy, resulting in cuff misses
Reduced Plunger Spring force5
- The Plunger spring force was reduced to eliminate Plunger rebound and improve needle-to-cuff engagement at the end of Step 2 (Depressing the Plunger to Deploy the Needles)
Lubrication added to the Cuffs of the Foot5
- Lubrication has been added to the anterior and posterior cuffs of the Foot to reduce the overall deployment force required to depress the Plunger and deploy the Needles.
What makes the needles in Perclose™ ProStyle™ stronger? What is the impact of stronger needles during deployment?
- The material of the anterior and posterior needles of Perclose™ ProStyle™ was changed from the normal Stainless Steel (used in Perclose ProGlide™) to a custom High Tensile Stainless Steel with 11-16% increased needle strength to minimize needle deflection and improve needle penetration through less compliant anatomy.5
- The higher tensile strength needles in Perclose™ ProStyle™ minimize needle deflection, reduce cuff misses and improve needle penetration through complex, challenging and less compliant anatomy.5
- During benchtop testing, Perclose™ ProStyle™ demonstrated a 15% improved success rate of suture deployment over that of Perclose ProGlide™ for better reliability and performance in more complex, challenging and less compliant anatomy.5
What features have been added to the Perclose™ ProStyle™ SMCR system to increase ease of use and ease of learning?
Matching Depth Reference Markers5
- New matching Depth Reference Markers have been added to the Perclose™ ProStyle™ device and Suture Trimmer to provide an optional visual estimation of the access site depth
- The distance between the center of each Depth Reference Marker is exactly 1 cm apart and are easy to see, thus improving ease of learning and ease of use
Guide Wire Exit Port5
- The Guide Wire Exit Port is the same as that of Perclose ProGlide™, however, the printing area surrounding the Guide Wire Exit Port has been increased by 329%
- This modification of the printing design improves visibility of the Guide Wire Exit Port and can improve the ease and efficiency of regaining guide wire access
QuickCut™5
- The opening area of QuickCut™ has been redesigned by increasing the suture capture area by approximately 39% to facilitate easier and more efficient suture capture and trimming of the suture at the end of Step 3 (Pull the Plunger back to deploy the Sutures)
Thumb Knob and Suture Gate5
- The Thumb Knob and Suture Gate on the updated Perclose™ ProStyle™ Suture Trimmer have been updated with matching white colors to improve ease of use and ease of learning when opening the Suture Gate
- When the white Thumb Knob is retracted, the white Suture Gate opens, providing a more intuitive experience when using the Suture Trimmer
Trimming Lever and Suture Trimmer Blade5
- The spring rate on the red Trimming Lever has been increased to reduce the potential for accidental trimming
- The Suture Trimmer Blade design was modified to remove a laser cut force reduction feature on the shaft, improving cutting efficiency and tactile feedback during trimming for improved ease of use
Perclose Snared Knot Pusher5
- This accessory knot advancement provides an alternative option to the Suture Trimmer with a tapered shaft for easier insertion, a longer working length for deeper tissue tracts, and concave tip that sits on top of the knot for more tactile knot advancement
White-tipped Non-Rail5
- The white marking on Non-Rail suture limb is created by using a shrink tubing on the end of the suture limb
- The amount of white pigment in the shrink tubing has been increased by 250% to provide better contrast and differentiation from the blue Rail suture limb and ease the visual identification of the white-tipped Non-Rail suture limb.
Additional hydrophilic coatings5
Perclose™ ProStyle™ device
- The tapered Sheaths of both Perclose ProGlide™ and Perclose™ ProStyle™ are coated with the same hydrophilic coating
- With Perclose™ ProStyle™, the same hydrophilic coating has been extended up to coat Distal Guide as well, to further ease device advancement by reducing the insertion force required when advancing the device into the body8
- During benchtop testing, the mean coefficient of kinetic friction at the Distal Guide was 27% lower for Perclose™ ProStyle™ compared to Perclose ProGlide™, allowing for improved ease of device advancement8
Perclose™ ProStyle™ Suture Trimmer
- A hydrophilic coating has also been added to the Sheath of the Perclose™ ProStyle™ Suture Trimmer for improved ease of use during knot advancement through the tissue tract8
Perclose™ ProStyle™ and Perclose ProGlide™, silhouette logo, device shape and appearance, lever, handle, color blue, color purple and related marks and/or designs are the intellectual property of the Abbott group of companies in various territories.
References
†As of September 2024, per global market share data on file at Abbott.
*As compared to Angio-Seal‡, MANTA‡, Celt ACD‡, ExoSeal‡, Mynx‡, Vascade‡. Data on file at Abbott.
- Perclose™ ProStyle™ SMCR System – Instructions for Use (IFU). Refer to IFU for additional information.
- Fabbricatore D et al. (2023) Ambulatory PV isolation workflow using suture-mediated vascular closure devices: a prospective observational cohort study. (PRO-PVI Study). Europace. 25(4):1361-1368
- Schneider, DB et al. Clinical and economic outcomes of ProGlide compared with surgical repair of large bore arterial access. Journal of comparative effectiveness research, [s. l.], v. 8, n. 16, p. 1381–1392, 2019.
- Kar S, Hermiller J et al. The use of Perclose ProGlide SMC System for Venous Access-Site Closure up to 24F Sheaths. CRT 2018 Poster.
- For arterial sheath sizes greater than 8F, at least two devices and the pre-close technique are required.
- For venous sheath sizes greater than 14F, at least two devices and the pre-close technique are required.
- Max. OD 26F/0.340 inches/8.62 mm; Max. OD 29F/0.378 inches/9.59 mm. Data on file at Abbott.
- Data on file at Abbott.
- July 2024 Finance Report. Data on file at Abbott.
- Primary intention healing occurs where vessel wall edges are brought together, adjacent to each other. This can be achieved with suture, stitches, staples and clips. Advances in Skin & Wound Care: Healing by Intention. Salcido, Richard. 2017.
MAT-2102358 v4.0
Perclose™ ProStyle™ Suture-Mediated Closure and Repair (SMCR) System

Indications:
The Perclose™ ProStyle™ Suture-Mediated Closure and Repair System is indicated for the percutaneous delivery of suture for closing the common femoral artery and vein access sites of patients who have undergone diagnostic or interventional catheterization procedures.
The Perclose™ ProStyle™ SMCR System is indicated for closing the common femoral vein in single or multiple access sites per limb.
The Perclose™ ProStyle™ SMCR System is used without or, if required, with adjunctive manual compression.
For access sites in the common femoral artery using 5F to 21F sheaths. For arterial sheath sizes greater than 8F, at least two devices and the pre-close technique are required.
For access sites in the common femoral vein using 5F to 24F sheaths. For venous sheath sizes greater than 14F, at least two devices and the pre-close technique are required.
Caution:
Federal law restricts this medical device to sale by or on the order of a physician (or allied healthcare professionals, authorized by, or under the direction of, such physicians) who is trained in diagnostic and / or interventional catheterization procedures and who has been trained by an authorized representative of Abbott.
Prior to use, the operator must review the Instructions for Use and be familiar with the deployment techniques associated with the use of this device.
During closure of access sites using a procedural sheath greater than 8F, it is recommended that a vascular surgeon or a surgeon with vascular training be available in case surgical conversion to control bleeding and to repair the vessel is needed.
Contraindications:
There are no known contraindications to the use of this device.
Warnings:
Do not use the Perclose™ ProStyle™ SMCR System if the packaging or sterile barrier has been previously opened or damaged or if the components appear to be damaged or defective.
DO NOT RESTERILIZE OR REUSE. The Perclose™ ProStyle™ SMCR System is intended for single use only.
Do not use the Perclose™ ProStyle™ SMCR System if the sterile field has been broken where bacterial contamination of the sheath or surrounding tissues may have occurred, since such a broken sterile field may result in infection.
Do not use the Perclose™ ProStyle™ SMCR System if the puncture site is located above the most inferior border of the inferior epigastric artery (IEA) and / or above the inguinal ligament based upon bony landmarks, since such a puncture site may result in a retroperitoneal hematoma. Perform a femoral angiogram to verify the location of the puncture site. Note: This may require both a right anterior oblique (RAO) and left anterior oblique (LAO) angiogram to adequately visualize where the sheath enters the femoral vessel.
Do not use the Perclose™ ProStyle™ SMCR System in arterial or venous access if the puncture is through the posterior wall or if there are multiple punctures in the same access site, since such punctures may result in a hematoma or retroperitoneal bleed.
Do not use the Perclose™ ProStyle™ SMCR System if the puncture site is located in the superficial femoral artery or the profunda femoris artery, or the bifurcation of these vessels, since such puncture sites may result in a pseudoaneurysm, intimal dissection, or an acute vessel closure (thrombosis of small artery lumen). Perform a femoral angiogram to verify the location of the puncture site. Note: This may require both a right anterior oblique (RAO) and left anterior oblique (LAO) angiogram to adequately visualize where the sheath enters the femoral vessel.
Precautions:
- Prior to use, inspect the Perclose™ ProStyle™ SMCR System to ensure that the sterile packaging has not been damaged during shipment. Examine all components prior to use to verify proper function. Exercise care during device handling to reduce the possibility of accidental device breakage.
- As with all catheter-based procedures, infection is a possibility. Observe sterile technique at all times when using the Perclose™ ProStyle™ SMCR System. Employ appropriate groin management, as per hospital protocol, post-procedure, and post-hospital discharge to prevent infection.
- Use a single wall puncture technique. Do not puncture the posterior wall of the vessel in arterial and venous access.
- Do not deploy the Perclose™ ProStyle™ Device at an elevated angle against resistance as this may cause a cuff miss or device breakage.
- There are no reaccess restrictions if previous arteriotomy / venotomy repairs were achieved with Abbott Medical SMC or SMCR systems.
- If significant blood flow is present around the Perclose™ ProStyle™ Device, do not deploy needles. Remove the device over a 0.038" (0.97 mm) (or smaller) guide wire and insert an appropriately sized sheath.
- Prior to depressing the plunger to advance the needles, stabilize the device by the body to ensure the foot is apposed to the vessel wall and the device does not twist during deployment. Twisting (torquing) of the device could lead to needle deflection resulting in a cuff miss. Do not use excessive force or repeatedly depress the plunger. Excessive force on the plunger during deployment could potentially cause breakage of the device, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- Do not apply excessive force to the lever when opening the foot and returning the foot to its original position down to the body of the device. Do not attempt to remove the device without closing the lever. Excessive force on the lever or attempting to remove the device without closing the lever could cause breakage of the device and / or lead to vessel trauma, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- Do not advance or withdraw the Perclose™ ProStyle™ Device against resistance until the cause of that resistance has been determined. Excessive force used to advance or torque the Perclose™ ProStyle™ Device should be avoided, as this may lead to significant vessel damage and / or breakage of the device, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- If excessive resistance in advancing the Perclose™ ProStyle™ Device is encountered, withdraw the device over a 0.038" (0.97 mm) (or smaller) guide wire and reinsert the introducer sheath or use manual compression.
- Remove the Perclose™ ProStyle™ sheath before tightening the suture. Failure to remove the sheath prior to tightening the suture may result in detachment of the tip of the sheath.
- Care should be taken to avoid damage to the suture from handling. Avoid crushing damage due to application of surgical instruments such as clamps, forceps or needle holders.
- For catheterization procedures using a 5F – 8F procedural sheath, use manual compression in the event that bleeding from the femoral access site persists after the use of the Perclose™ ProStyle™ SMCR System to obtain hemostasis.
- For catheterization procedures using a procedural sheath > 8F, use manual compression, compression assisted devices, surgical repair, and / or other appropriate treatment methods in the event that bleeding from the femoral access site persists after the use of the Perclose™ ProStyle™ SMCR System to obtain hemostasis.
- For catheterization procedures using a procedural sheath > 8F, where the operating physician is not a vascular surgeon, it is recommended that a vascular surgeon or a surgeon with vascular training be available during the procedure to perform any necessary vascular surgical intervention.
- If the Perclose™ ProStyle™ Device is used to close and repair multiple access sites in the same vessel, space the access sites apart adequately to minimize sheath-device interference.
Potential Adverse Events:
Potential adverse events associated with use of vessel closure devices may include, but are not limited to, the following:
- Allergic reaction or hypersensitivity to device components
- Vascular access complications which may require transfusion or vessel repair, including:
- Anemia
- Aneurysm
- Arteriovenous fistula
- Bleeding / hemorrhage / re-bleeding
- Bruising
- Hematoma
- Embolism
- Inflammation
- Intimal tear / dissection
- Perforation
- Pseudoaneurysm
- Retroperitoneal hematoma / bleeding
- Scar formation
- Wound dehiscence
- Cardiac arrhythmias (including conduction disorders, atrial and ventricular arrhythmias)
- Atrial arrhythmias
- Ventricular arrhythmias
- Femoral artery / venous complications which may require additional intervention, including:
- Arterial / venous stenosis
- Arterial / venous occlusion
- Arteriovenous fistula
- Intimal tear / dissection
- Ischemia distal to closure site
- Nerve injury
- Numbness
- Thrombus formation
- Vascular injury
- Venous thromboembolism (including deep vein thrombosis, pulmonary embolism, post-procedure pulmonary embolism)
- Infection - local or systemic
- Pain
- Hemodynamic instability:
- Hypotension / hypertension
- Vasovagal episode
- Death
- Device complications
- Device failure
- Device malfunction
MAT-2100368 v4.0
Perclose ProGlide™ Suture-Mediated Closure (SMC) System

Indications:
The Perclose ProGlide™ Suture-Mediated Closure System is indicated for the percutaneous delivery of suture for closing the common femoral artery and vein access site of patients who have undergone diagnostic or interventional catheterization procedures.
The Perclose ProGlide™ SMC System is indicated for closing the common femoral vein in single or multiple access sites per limb.
The Perclose ProGlide™ SMC System is used without or, if required, with adjunctive manual compression.
For access sites in the common femoral artery using 5F to 21F sheaths. For arterial sheath sizes greater than 8F, at least two devices and the pre-close technique are required.
For access sites in the common femoral vein using 5F to 24F sheaths. For venous sheath sizes greater than 14F, at least two devices and the pre-close technique are required.
Caution:
Federal law restricts this medical device to sale by or on the order of a physician (or allied healthcare professionals, authorized by, or under the direction of, such physicians) who is trained in diagnostic and / or interventional catheterization procedures and who has been trained by an authorized representative of Abbott.
Prior to use, the operator must review the Instructions for Use and be familiar with the deployment techniques associated with the use of this device.
During closure of access sites using a procedural sheath greater than 8F, it is recommended that a vascular surgeon or a surgeon with vascular training be available in case surgical conversion to control bleeding and to repair the vessel is needed.
Contraindications:
There are no known contraindications to the use of this device.
Warnings:
Do not use the Perclose ProGlide™ SMC System if the packaging or sterile barrier has been previously opened or damaged or if the components appear to be damaged or defective.
DO NOT RESTERILIZE OR REUSE. The Perclose ProGlide™ SMC System is intended for single use only.
Do not use the Perclose ProGlide™ SMC System if the sterile field has been broken where bacterial contamination of the sheath or surrounding tissues may have occurred, since such a broken sterile field may result in infection.
Do not use the Perclose ProGlide™ SMC System if the puncture site is located above the most inferior border of the inferior epigastric artery (IEA) and / or above the inguinal ligament based upon bony landmarks, since such a puncture site may result in a retroperitoneal hematoma. Perform a femoral angiogram to verify the location of the puncture site. Note: This may require both a right anterior oblique (RAO) and left anterior oblique (LAO) angiogram to adequately visualize where the sheath enters the femoral vessel.
Do not use the Perclose ProGlide™ SMC System in arterial or venous access if the puncture is through the posterior wall or if there are multiple punctures in the same access site, since such punctures may result in a hematoma or retroperitoneal bleed.
Do not use the Perclose ProGlide™ SMC System if the puncture site is located in the superficial femoral artery or the profunda femoris artery, or the bifurcation of these vessels, since such puncture sites may result in a pseudoaneurysm, intimal dissection, or an acute vessel closure (thrombosis of small artery lumen). Perform a femoral angiogram to verify the location of the puncture site. Note: This may require both a right anterior oblique (RAO) and left anterior oblique (LAO) angiogram to adequately visualize where the sheath enters the femoral vessel.
Precautions:
- Prior to use, inspect the Perclose ProGlide™ SMC System to ensure that the sterile packaging has not been damaged during shipment. Examine all components prior to use to verify proper function. Exercise care during device handling to reduce the possibility of accidental device breakage.
- As with all catheter-based procedures, infection is a possibility. Observe sterile technique at all times when using the Perclose ProGlide™ SMC System. Employ appropriate groin management, as per hospital protocol, post procedure and post hospital discharge to prevent infection.
- Use a single wall puncture technique. Do not puncture the posterior wall of the vessel in arterial and venous access.
- Do not deploy the Perclose ProGlide™ Device at an elevated angle against resistance as this may cause a cuff miss or device breakage.
- There are no reaccess restrictions if previous arteriotomy / venotomy repairs were achieved with Abbott Medical SMC or SMCR systems.
- If significant blood flow is present around the Perclose ProGlide™ Device, do not deploy needles. Remove the device over a 0.038" (0.97 mm) (or smaller) guide wire and insert an appropriately sized sheath.
- Prior to depressing the plunger to advance the needles, stabilize the device by the body to ensure the foot is apposed to the vessel wall and the device does not twist during deployment. Twisting (torquing) of the device could lead to needle deflection resulting in a cuff miss. Do not use excessive force or repeatedly depress the plunger. Excessive force on the plunger during deployment could potentially cause breakage of the device, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- Do not apply excessive force to the lever when opening the foot and returning the foot to its original position down to the body of the device. Do not attempt to remove the device without closing the lever. Excessive force on the lever or attempting to remove the device without closing the lever could cause breakage of the device and / or lead to vessel trauma, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- Do not advance or withdraw the Perclose ProGlide™ Device against resistance until the cause of that resistance has been determined. Excessive force used to advance or torque the Perclose ProGlide™ Device should be avoided, as this may lead to significant vessel damage and / or breakage of the device, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- If excessive resistance in advancing the Perclose ProGlide™ Device is encountered, withdraw the device over a 0.038" (0.97 mm) (or smaller) guide wire and reinsert the introducer sheath or use manual compression.
- Remove the Perclose ProGlide™ sheath before tightening the suture. Failure to remove the sheath prior to tightening the suture may result in detachment of the tip of the sheath.
- Care should be taken to avoid damage to the suture from handling. Avoid crushing damage due to application of surgical instruments such as clamps, forceps or needle holders.
- For catheterization procedures using a 5F – 8F procedural sheath, use manual compression in the event that bleeding from the femoral access site persists after the use of the Perclose ProGlide™ SMC System to obtain hemostasis.
- For catheterization procedures using a procedural sheath > 8F, use manual compression, compression assisted devices, surgical repair, and / or other appropriate treatment methods in the event that bleeding from the femoral access site persists after the use of the Perclose ProGlide™ SMC System to obtain hemostasis.
- For catheterization procedures using a procedural sheath > 8F, where the operating physician is not a vascular surgeon, it is recommended that a vascular surgeon or a surgeon with vascular training be available during the procedure to perform any necessary vascular surgical intervention.
- If the Perclose ProGlide™ Device is used to close and repair multiple access sites in the same vessel, space the access sites apart adequately to minimize sheath-device interference
Potential Adverse Events:
Potential adverse events associated with use of vessel closure devices may include, but are not limited to, the following:
- Allergic reaction or hypersensitivity to device components
- Vascular access complications which may require transfusion or vessel repair, including:
- Anemia
- Aneurysm
- Arteriovenous fistula
- Bleeding / hemorrhage / re-bleeding
- Bruising
- Hematoma
- Embolism
- Inflammation
- Intimal tear / dissection
- Perforation
- Pseudoaneurysm
- Retroperitoneal hematoma / bleeding
- Scar formation
- Wound dehiscence
- Cardiac arrhythmias (including conduction disorders, atrial and ventricular arrhythmias)
- Atrial arrhythmias
- Ventricular arrhythmias
- Femoral artery / venous complications which may require additional intervention, including:
- Arterial / venous stenosis
- Arterial / venous occlusion
- Arteriovenous fistula
- Intimal tear / dissection
- Ischemia distal to closure site
- Nerve injury
- Numbness
- Thrombus formation
- Vascular injury
- Venous thromboembolism (including deep vein thrombosis, pulmonary embolism, post-procedure pulmonary embolism)
- Infection – local or systemic
- Pain
- Hemodynamic instability:
- Hypotension / hypertension
- Vasovagal episode
- Death
- Device complications
- Device failure
- Device malfunction
MAT-2100358 v4.0
