Benefit From Improved Lab Efficiency
We understand how things may not always go as planned during a procedure.
Unexpected access-site related complications can be costly and wreak havoc on your schedule for the day.
You already spend your entire day helping patients. You and your team deserve to know when you'll be going home at the end of the day!
Commit to a consistent, efficient, and predictable close.

Facilitate quick turnaround times between cases.

Feel the satisfaction after completing your full day's work.

The Perclose™ Difference
The Perclose™ Family of Products offer a COMPREHENSIVE, VERSATILE, and PROVEN solution for hemostasis management unrivaled by any other single offering.
That's why Perclose™ Devices are the world's leading vascular closure system.†

Comprehensive
Breadth of Transfemoral Applications
Perclose™ Devices offer you broadest indications* on the market allowing for use across the widest range of transfemoral procedures.5-41
- Arterial Sheaths 5-21F1
(Max OD 26F)2 - Venous Sheaths 5-24F1
(Max OD 29F)2
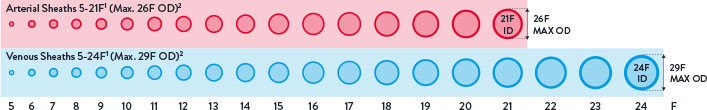
Max. OD 26F/0.340 inches/8.62 mm; Max. OD 29F/0.378 inches/9.59 mm. Data on file at Abbott.
What our Customers are Saying...
Versatile
Keep Your Options Open
Before the Procedure
Customize your closing approach by deploying the sutures before the procedure (pre-close) or after the procedure, by using one or more devices per access site, or by rotating your angle of needle deployment to avoid any challenging anatomy.1
During the Procedure
Maintain guide wire access even after deployment of the Perclose™ sutures to keep all therapeutic options open.1
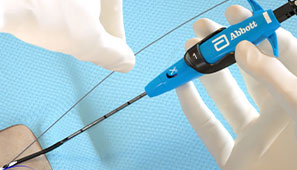
After the Procedure
Pull the sheath sooner due to no ACT-level requirements, and re-puncture the same access site immediately or in the near-term since Perclose™ Devices have no reaccess restrictions.1
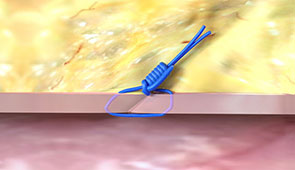
What our Customers are Saying...
Proven
Established Decades-Long Track Record
20,000,000+
closures and counting across 100+ countries worldwide2,3
> 30 Years
of Perclose™ Legacy as the pioneers of suture-mediated closure4
Years of clinical evidence featuring successful closure with Perclose™ products across a wide range of transfemoral procedures:
Percutaneous Coronary Intervention (PCI)5-7
Transcatheter Edge-to-Edge Repair (TEER, TMVr)8-10
Transcatheter Aortic Valve Implantation (TAVI)11-18
Endovascular Aortic Repairs (PEVAR/TEVAR)18-22
Structural Heart Occluders (LAAO, PFO, ASD)23-25
EP Catheter Ablations and Leadless Pacemakers26-33
Vascular Interventional Radiology34-35
Mechanical Circulatory Support36-38
What our Customers are Saying...
Getting Started on your Perclose™ Journey
Contact a Specialist
Identify which of the following transfemoral applications apply to your practice:
- Arterial access
- Venous access
- Small-bore access sites (≤8F)
- Large-bore access sites (>8F)
- Multiple access sites in the same vessel (EP)
Get Certified
Complete Abbott's official training program for the Perclose™ Family of Products to learn the following:
- Single Device Deployment
- Double Perclose Technique for Large-Bore Closure
- The Pre-Close Technique vs. Post-Close Technique
- Tips and techniques for troubleshooting
Choose Efficiency
Make the decision to standardize your approach to hemostasis management by choosing a comprehensive, versatile, and proven vascular closure system that meets the majority of your transfemoral procedure needs.
Don't Just Close it. Perclose™ it.
The Perclose™ Family of Vascular Closure Systems
Perclose™ ProStyle™ Suture-Mediated Closure and Repair (SMCR) System
The newest generation of the Perclose™ Family of Vascular Closure Systems designed with high-tensile strength needles for increased reliability and a more intuitive deployment experience compared to the older Perclose ProGlide™ SMC System.2

Perclose ProGlide™ Suture-Mediated Closure (SMC) System
The choice for suture-mediated closure in countries where the Perclose™ ProStyle™ SMCR System has not yet been launched.
Other Hemostasis Management Solutions
Abbott also offers the following hemostasis management solutions for situations where Perclose™ Devices may not be preferred for the specific clinical situation.
StarClose SE™ Vascular Closure System (VCS)
The novel nitinol clip-based vascular closure system that achieves hemostasis for 5-6F arterial access sites for a truly-extravascular close.2,39
Prostar XL™ Percutaneous Vascular Surgical System (PVSS)
The large-bore vascular closure system, indicated for 8.5-10F arterial access sites that deliver two braided sutures with a "needles out" approach.2,40
FemoStop™ Gold Femoral Compression System
Offers hands-free compression of the femoral artery or vein to offer precise hemostasis management and patient comfort after transfemoral interventions.41
Perclose™ ProStyle™ and Perclose ProGlide™, silhouette logo, device shape and appearance, lever, handle, color blue, color purple and related marks and/or designs are the intellectual property of the Abbott group of companies in various territories.
References:
As of September 2024, per global market share data on file at Abbott.
*As compared to Angio-Seal‡, ExoSeal‡, FemoSeal‡, InClosure‡, MANTA‡, Mynx‡, PerQseal‡, Vascade‡, Velox CD‡, X-Seal‡. Data on file at Abbott.
Perclose™ Suture-Mediated Vascular Closure Systems
1. Perclose™ ProStyle™ SMCR System and Perclose ProGlide™ SMC System – Instructions for Use (IFU). Refer to IFU for additional information.
2. Data on file at Abbott.
3. July 2024 Finance Report. Data on file at Abbott.
4. On Nov. 8, 1993, the first (Perclose) patent was filed for the percutaneous suture vascular closure device.
Percutaneous Coronary Interventions (PCI)
5. VARIS, E.; GURBUZ, D. C. Learning Curve of Perclose ProGlide Utilization During Percutaneous Coronary Intervention. Cureus, [s. l.], v. 15, n. 4, p. e38155, 2023.
6. NIKOLSKY, E. et al. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures: A meta-analysis. Journal of the American College of Cardiology (JACC), [s. l.], v. 44, n. 6, p. 1200–1209, 2004.
7. BHATT, D. L. et al. Successful “pre-closure” of 7Fr and 8Fr femoral arteriotomies with a 6Fr suture-based device (the Multicenter Interventional Closer Registry). The American journal of cardiology, [s. l.], v. 89, n. 6, p. 777–779, 2002.
Mitral Transcatheter Edge-to-Edge Repair (TEER)
8. MOHAMMED, M. et al. Preclosure of large bore venous access sites in patients undergoing transcatheter mitral replacement and repair. Catheterization & Cardiovascular Interventions, [s. l.], v. 100, n. 1, p. 163–168, 2022. (TEER)
9. GEIS, N. A. et al. Feasibility and clinical benefit of a suture-mediated closure device for femoral vein access after percutaneous edge-to-edge mitral valve repair. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology, [s. l.], v. 10, n. 11, p. 1346–1353, 2015. (TEER)
10. KAR S, Hermiller J et al. The use of Perclose ProGlide SMC System for Venous Access-Site Closure up to 24F Sheaths. CRT 2018 Poster.
Transcatheter Aortic Valve Implantation/ Replacement (TAVI/ TAVR)
11. DRAFTS, B. C. et al. Comparison of outcomes with surgical cut-down versus percutaneous transfemoral transcatheter aortic valve replacement: TAVR transfemoral access comparisons between surgical cut-down and percutaneous approach. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions, [s. l.], v. 91, n. 7, p. 1354–1362, 2018.
12. MCCABE, J. M. et al. Surgical Versus Percutaneous Femoral Access for Delivery of Large-Bore Cardiovascular Devices (from the PARTNER Trial). The American journal of cardiology, [s. l.], v. 117, n. 10, p. 1643–1650, 2016.
13. DENCKER, D. et al. Frequency and Effect of Access-Related Vascular Injury and Subsequent Vascular Intervention After Transcatheter Aortic Valve Replacement. The American journal of cardiology, [s. l.], v. 118, n. 8, p. 1244–1250, 2016.
14. BARBASH, I. M. et al. Comparison of vascular closure devices for access site closure after transfemoral aortic valve implantation. European heart journal, [s. l.], v. 36, n. 47, p. 3370–3379, 2015.
15. DIMITRIADIS, Z. et al. Impact of closure devices on vascular complication and mortality rates in TAVI procedures. International Journal of Cardiology, [s. l.], v. 241, p. 133–137, 2017.
16. BARBASH, I. M. et al. Comparison of MANTA versus Perclose Prostyle large-bore vascular closure devices during transcatheter aortic valve implantation. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions, [s. l.], v. 103, n. 1, p. 160–168, 2024.
17. HOFFMANN P et al. Access site complications after transfemoral aortic valve implantation - a comparison of Manta and ProGlide. CVIR Endovascular, [s. l.], v. 1, n. 1, p. 1–8, 2018.
18. SCHNEIDER, D. B. et al. Clinical and economic outcomes of ProGlide compared with surgical repair of large bore arterial access. Journal of comparative effectiveness research, [s. l.], v. 8, n. 16, p. 1381–1392, 2019.
Endovascular Aortic Repair (PEVAR/ TEVAR)
19. PRATESI, G. et al. Italian Percutaneous EVAR (IPER) Registry: outcomes of 2381 percutaneous femoral access sites’ closure for aortic stent-graft. The Journal of cardiovascular surgery, [s. l.], v. 56, n. 6, p. 889–898, 2015.
20. NELSON, P. R. et al. A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). Journal of Vascular Surgery, [s. l.], v. 59, n. 5, p. 1181–1193, 2014.
21. KRAJCER, Z.; RAMAIAH, V.; HUETTER, M. Fast-track endovascular aneurysm repair: rationale and design of the multicenter Least Invasive Fast-Track EVAR (LIFE) registry. BMC cardiovascular disorders, [s. l.], v. 15, p. 174, 2015. 8. SAADI, E. K. et al. Totally Percutaneous Access Using Perclose Proglide for Endovascular Treatment of Aortic Diseases. Brazilian Journal of Cardiovascular Surgery, [s. l.], v. 32, n. 1, 2017.
22. ROCHE-NAGLE, G.; HAZEL, M.; RAJAN, D. K. Financial Impact of PEVAR Compared With Standard Endovascular Repair in Canadian Hospitals. Canadian Association of Radiologists Journal, [s. l.], v. 69, n. 2, p. 215–219, 2018.
Structural Heart Occluders (LAAO, PFO, ASD)
23. LODHI, H. et al. Comparison of Figure-of-Eight Suture and Perclose ProGlide Suture-Mediated Closure in Large Bore Venous Access Hemostasis: A Randomized Controlled Trial. The American journal of cardiology, [s. l.], v. 209, p. 181–183, 2023.
24. STEINER, K. et al. Same-day discharge after percutaneous closure of persistent foramen ovale using intracardiac echocardiography and the Gore Septal Occluder. Frontiers in cardiovascular medicine, [s. l.], v. 11, p. 1408543, 2024.
25. HAN, X. et al. Perclose ProGlide devices simplified the removal of the femoral venous cannulas for the transcatheter closure of atrial septal defect: A single-center retrospective study. Vascular Investigation & Therapy, [s. l.], v. 5, n. 2, p. 31–36, 2022.
Cardiac Ablations (RF, Cryo, PFA)
26. FABBRICATORE, D. et al. Ambulatory pulmonary vein isolation workflow using the Perclose ProGlide™ suture-mediated vascular closure device: the PRO-PVI study. EP: Europace, [s. l.], v. 25, n. 4, p. 1361–1368, 2023.
27. KIANI, S. et al. Percutaneous Vascular Closure Compared With Manual Compression in Atrial Fibrillation Ablation. JACC. Clinical electrophysiology, [s. l.], v. 8, n. 6, p. 803–805, 2022.
28. VERMA, S. et al, Feasibility and Safety of Same Day Discharge for Patients Undergoing Atrial Fibrillation (AF) Ablation in a Community Hospital Setting. HRS 2020 Science Online, May 2020.
29. SUN, J.-Y. et al. Feasibility and clinical benefits of the double-ProGlide technique for hemostasis after cryoballoon atrial fibrillation ablation with uninterrupted oral anticoagulants. Journal of geriatric cardiology : JGC, [s. l.], v. 20, n. 4, p. 268–275, 2023.
30. TILZ, R. R. et al. Venous vascular closure system vs. figure-of-eight suture following atrial fibrillation ablation: the STYLE-AF Study. EP: Europace, [s. l.], v. 26, n. 5, p. 1–12, 2024.
31. KIANI, S. et al. Costs, efficiency, and patient-reported outcomes associated with suture-mediated percutaneous closure for atrial fibrillation ablation: Secondary analysis of a randomized clinical trial. Journal of cardiovascular electrophysiology, [s. l.], 2024.
Leadless Pacemakers
32. DESHMUKH, A. et al. Double ProGlide preclose technique for vascular access closure after leadless pacemaker implantation. Journal of Interventional Cardiac Electrophysiology, [s. l.], v. 63, n. 2, p. 341–343, 2022.
33. RODRIGUEZ-TAVERAS, J. et al. Double Perclose increases the efficiency of leadless pacemaker implantation: A propensity score–matched analysis. Heart Rhythm O2, [s. l.], v. 5, n. 10, p. 750–753, 2024.
Vascular Interventional Radiology
34. JENSEN, J. et al. The Inflammatory Response to Femoral Arterial Closure Devices: A Randomized Comparison Among FemoStop, AngioSeal, and Perclose. CardioVascular & Interventional Radiology, [s. l.], v. 31, n. 4, p. 751–755, 2008.
35. HENK, C. B. et al. ‘The Closer’-percutaneous vascular suture device: evaluation of safety and performance in neuroangiography. European Journal of Radiology, [s. l.], v. 48, n. 3, p. 237–243, 2003.
36. LATA, K. et al. Pre-close technique of percutaneous closure for delayed hemostasis of large-bore femoral sheaths. Journal of Interventional Cardiology, [s. l.], v. 31, n. 4, p. 504–510, 2018.
37. UNOKI, T. et al. Efficacy and safety of post-closure technique using Perclose ProGlide/ProStyle device for large-bore mechanical circulatory support access sites. Cardiovascular Revascularization Medicine, [s. l.], v. 62
38. SUN, G. et al. Outcomes comparison between percutaneous decannulation with perclose ProGlide and surgical decannulation of veno-arterial extracorporeal membrane oxygenation. Perfusion, [s. l.], p. 1, 2023.
Other Hemostasis Management Solutions IFUs
39. StarClose SE™ VCS System - Instructions for Use (IFU). Refer to IFU for additional information.
40. Prostar XL™ PVS System - Instructions for Use (IFU). Refer to IFU for additional information.
41. FemoStop™ Gold Femoral Compression System - Instructions for Use (IFU). Refer to IFU for additional information
MAT-2009415 v5.0
IMPORTANT SAFETY INFORMATION
Perclose™ ProStyle™ Suture-Mediated Closure and Repair (SMCR) System

Indications:
The Perclose™ ProStyle™ Suture-Mediated Closure and Repair System is indicated for the percutaneous delivery of suture for closing the common femoral artery and vein access sites of patients who have undergone diagnostic or interventional catheterization procedures.
The Perclose™ ProStyle™ SMCR System is indicated for closing the common femoral vein in single or multiple access sites per limb.
The Perclose™ ProStyle™ SMCR System is used without or, if required, with adjunctive manual compression.
For access sites in the common femoral artery using 5F to 21F sheaths. For arterial sheath sizes greater than 8F, at least two devices and the pre-close technique are required.
For access sites in the common femoral vein using 5F to 24F sheaths. For venous sheath sizes greater than 14F, at least two devices and the pre-close technique are required.
Caution:
Federal law restricts this medical device to sale by or on the order of a physician (or allied healthcare professionals, authorized by, or under the direction of, such physicians) who is trained in diagnostic and / or interventional catheterization procedures and who has been trained by an authorized representative of Abbott.
Prior to use, the operator must review the Instructions for Use and be familiar with the deployment techniques associated with the use of this device.
During closure of access sites using a procedural sheath greater than 8F, it is recommended that a vascular surgeon or a surgeon with vascular training be available in case surgical conversion to control bleeding and to repair the vessel is needed.
Contraindications:
There are no known contraindications to the use of this device.
Warnings:
Do not use the Perclose™ ProStyle™ SMCR System if the packaging or sterile barrier has been previously opened or damaged or if the components appear to be damaged or defective.
DO NOT RESTERILIZE OR REUSE. The Perclose™ ProStyle™ SMCR System is intended for single use only.
Do not use the Perclose™ ProStyle™ SMCR System if the sterile field has been broken where bacterial contamination of the sheath or surrounding tissues may have occurred, since such a broken sterile field may result in infection.
Do not use the Perclose™ ProStyle™ SMCR System if the puncture site is located above the most inferior border of the inferior epigastric artery (IEA) and / or above the inguinal ligament based upon bony landmarks, since such a puncture site may result in a retroperitoneal hematoma. Perform a femoral angiogram to verify the location of the puncture site. Note: This may require both a right anterior oblique (RAO) and left anterior oblique (LAO) angiogram to adequately visualize where the sheath enters the femoral vessel.
Do not use the Perclose™ ProStyle™ SMCR System in arterial or venous access if the puncture is through the posterior wall or if there are multiple punctures in the same access site, since such punctures may result in a hematoma or retroperitoneal bleed.
Do not use the Perclose™ ProStyle™ SMCR System if the puncture site is located in the superficial femoral artery or the profunda femoris artery, or the bifurcation of these vessels, since such puncture sites may result in a pseudoaneurysm, intimal dissection, or an acute vessel closure (thrombosis of small artery lumen). Perform a femoral angiogram to verify the location of the puncture site. Note: This may require both a right anterior oblique (RAO) and left anterior oblique (LAO) angiogram to adequately visualize where the sheath enters the femoral vessel.
Precautions:
- Prior to use, inspect the Perclose™ ProStyle™ SMCR System to ensure that the sterile packaging has not been damaged during shipment. Examine all components prior to use to verify proper function. Exercise care during device handling to reduce the possibility of accidental device breakage.
- As with all catheter-based procedures, infection is a possibility. Observe sterile technique at all times when using the Perclose™ ProStyle™ SMCR System. Employ appropriate groin management, as per hospital protocol, post-procedure, and post-hospital discharge to prevent infection.
- Use a single wall puncture technique. Do not puncture the posterior wall of the vessel in arterial and venous access.
- Do not deploy the Perclose™ ProStyle™ Device at an elevated angle against resistance as this may cause a cuff miss or device breakage.
- There are no reaccess restrictions if previous arteriotomy / venotomy repairs were achieved with Abbott Medical SMC or SMCR systems.
- If significant blood flow is present around the Perclose™ ProStyle™ Device, do not deploy needles. Remove the device over a 0.038" (0.97 mm) (or smaller) guide wire and insert an appropriately sized sheath.
- Prior to depressing the plunger to advance the needles, stabilize the device by the body to ensure the foot is apposed to the vessel wall and the device does not twist during deployment. Twisting (torquing) of the device could lead to needle deflection resulting in a cuff miss. Do not use excessive force or repeatedly depress the plunger. Excessive force on the plunger during deployment could potentially cause breakage of the device, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- Do not apply excessive force to the lever when opening the foot and returning the foot to its original position down to the body of the device. Do not attempt to remove the device without closing the lever. Excessive force on the lever or attempting to remove the device without closing the lever could cause breakage of the device and / or lead to vessel trauma, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- Do not advance or withdraw the Perclose™ ProStyle™ Device against resistance until the cause of that resistance has been determined. Excessive force used to advance or torque the Perclose™ ProStyle™ Device should be avoided, as this may lead to significant vessel damage and / or breakage of the device, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- If excessive resistance in advancing the Perclose™ ProStyle™ Device is encountered, withdraw the device over a 0.038" (0.97 mm) (or smaller) guide wire and reinsert the introducer sheath or use manual compression.
- Remove the Perclose™ ProStyle™ sheath before tightening the suture. Failure to remove the sheath prior to tightening the suture may result in detachment of the tip of the sheath.
- Care should be taken to avoid damage to the suture from handling. Avoid crushing damage due to application of surgical instruments such as clamps, forceps or needle holders.
- For catheterization procedures using a 5F – 8F procedural sheath, use manual compression in the event that bleeding from the femoral access site persists after the use of the Perclose™ ProStyle™ SMCR System to obtain hemostasis.
- For catheterization procedures using a procedural sheath > 8F, use manual compression, compression assisted devices, surgical repair, and / or other appropriate treatment methods in the event that bleeding from the femoral access site persists after the use of the Perclose™ ProStyle™ SMCR System to obtain hemostasis.
- For catheterization procedures using a procedural sheath > 8F, where the operating physician is not a vascular surgeon, it is recommended that a vascular surgeon or a surgeon with vascular training be available during the procedure to perform any necessary vascular surgical intervention.
- If the Perclose™ ProStyle™ Device is used to close and repair multiple access sites in the same vessel, space the access sites apart adequately to minimize sheath-device interference.
Potential Adverse Events:
Potential adverse events associated with use of vessel closure devices may include, but are not limited to, the following:
- Allergic reaction or hypersensitivity to device components
- Vascular access complications which may require transfusion or vessel repair, including:
- Anemia
- Aneurysm
- Arteriovenous fistula
- Bleeding / hemorrhage / re-bleeding
- Bruising
- Hematoma
- Embolism
- Inflammation
- Intimal tear / dissection
- Perforation
- Pseudoaneurysm
- Retroperitoneal hematoma / bleeding
- Scar formation
- Wound dehiscence
- Cardiac arrhythmias (including conduction disorders, atrial and ventricular arrhythmias)
- Atrial arrhythmias
- Ventricular arrhythmias
- Femoral artery / venous complications which may require additional intervention, including:
- Arterial / venous stenosis
- Arterial / venous occlusion
- Arteriovenous fistula
- Intimal tear / dissection
- Ischemia distal to closure site
- Nerve injury
- Numbness
- Thrombus formation
- Vascular injury
- Venous thromboembolism (including deep vein thrombosis, pulmonary embolism, post-procedure pulmonary embolism)
- Infection - local or systemic
- Pain
- Hemodynamic instability:
- Hypotension / hypertension
- Vasovagal episode
- Death
- Device complications
- Device failure
- Device malfunction
MAT-2100368 v4.0
Perclose ProGlide™ Suture-Mediated Closure (SMC) System

Indications:
The Perclose ProGlide™ Suture-Mediated Closure System is indicated for the percutaneous delivery of suture for closing the common femoral artery and vein access site of patients who have undergone diagnostic or interventional catheterization procedures.
The Perclose ProGlide™ SMC System is indicated for closing the common femoral vein in single or multiple access sites per limb.
The Perclose ProGlide™ SMC System is used without or, if required, with adjunctive manual compression.
For access sites in the common femoral artery using 5F to 21F sheaths. For arterial sheath sizes greater than 8F, at least two devices and the pre-close technique are required.
For access sites in the common femoral vein using 5F to 24F sheaths. For venous sheath sizes greater than 14F, at least two devices and the pre-close technique are required.
Caution:
Federal law restricts this medical device to sale by or on the order of a physician (or allied healthcare professionals, authorized by, or under the direction of, such physicians) who is trained in diagnostic and / or interventional catheterization procedures and who has been trained by an authorized representative of Abbott.
Prior to use, the operator must review the Instructions for Use and be familiar with the deployment techniques associated with the use of this device.
During closure of access sites using a procedural sheath greater than 8F, it is recommended that a vascular surgeon or a surgeon with vascular training be available in case surgical conversion to control bleeding and to repair the vessel is needed.
Contraindications:
There are no known contraindications to the use of this device.
Warnings:
Do not use the Perclose ProGlide™ SMC System if the packaging or sterile barrier has been previously opened or damaged or if the components appear to be damaged or defective.
DO NOT RESTERILIZE OR REUSE. The Perclose ProGlide™ SMC System is intended for single use only.
Do not use the Perclose ProGlide™ SMC System if the sterile field has been broken where bacterial contamination of the sheath or surrounding tissues may have occurred, since such a broken sterile field may result in infection.
Do not use the Perclose ProGlide™ SMC System if the puncture site is located above the most inferior border of the inferior epigastric artery (IEA) and / or above the inguinal ligament based upon bony landmarks, since such a puncture site may result in a retroperitoneal hematoma. Perform a femoral angiogram to verify the location of the puncture site. Note: This may require both a right anterior oblique (RAO) and left anterior oblique (LAO) angiogram to adequately visualize where the sheath enters the femoral vessel.
Do not use the Perclose ProGlide™ SMC System in arterial or venous access if the puncture is through the posterior wall or if there are multiple punctures in the same access site, since such punctures may result in a hematoma or retroperitoneal bleed.
Do not use the Perclose ProGlide™ SMC System if the puncture site is located in the superficial femoral artery or the profunda femoris artery, or the bifurcation of these vessels, since such puncture sites may result in a pseudoaneurysm, intimal dissection, or an acute vessel closure (thrombosis of small artery lumen). Perform a femoral angiogram to verify the location of the puncture site. Note: This may require both a right anterior oblique (RAO) and left anterior oblique (LAO) angiogram to adequately visualize where the sheath enters the femoral vessel.
Precautions:
- Prior to use, inspect the Perclose ProGlide™ SMC System to ensure that the sterile packaging has not been damaged during shipment. Examine all components prior to use to verify proper function. Exercise care during device handling to reduce the possibility of accidental device breakage.
- As with all catheter-based procedures, infection is a possibility. Observe sterile technique at all times when using the Perclose ProGlide™ SMC System. Employ appropriate groin management, as per hospital protocol, post procedure and post hospital discharge to prevent infection.
- Use a single wall puncture technique. Do not puncture the posterior wall of the vessel in arterial and venous access.
- Do not deploy the Perclose ProGlide™ Device at an elevated angle against resistance as this may cause a cuff miss or device breakage.
- There are no reaccess restrictions if previous arteriotomy / venotomy repairs were achieved with Abbott Medical SMC or SMCR systems.
- If significant blood flow is present around the Perclose ProGlide™ Device, do not deploy needles. Remove the device over a 0.038" (0.97 mm) (or smaller) guide wire and insert an appropriately sized sheath.
- Prior to depressing the plunger to advance the needles, stabilize the device by the body to ensure the foot is apposed to the vessel wall and the device does not twist during deployment. Twisting (torquing) of the device could lead to needle deflection resulting in a cuff miss. Do not use excessive force or repeatedly depress the plunger. Excessive force on the plunger during deployment could potentially cause breakage of the device, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- Do not apply excessive force to the lever when opening the foot and returning the foot to its original position down to the body of the device. Do not attempt to remove the device without closing the lever. Excessive force on the lever or attempting to remove the device without closing the lever could cause breakage of the device and / or lead to vessel trauma, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- Do not advance or withdraw the Perclose ProGlide™ Device against resistance until the cause of that resistance has been determined. Excessive force used to advance or torque the Perclose ProGlide™ Device should be avoided, as this may lead to significant vessel damage and / or breakage of the device, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- If excessive resistance in advancing the Perclose ProGlide™ Device is encountered, withdraw the device over a 0.038" (0.97 mm) (or smaller) guide wire and reinsert the introducer sheath or use manual compression.
- Remove the Perclose ProGlide™ sheath before tightening the suture. Failure to remove the sheath prior to tightening the suture may result in detachment of the tip of the sheath.
- Care should be taken to avoid damage to the suture from handling. Avoid crushing damage due to application of surgical instruments such as clamps, forceps or needle holders.
- For catheterization procedures using a 5F – 8F procedural sheath, use manual compression in the event that bleeding from the femoral access site persists after the use of the Perclose ProGlide™ SMC System to obtain hemostasis.
- For catheterization procedures using a procedural sheath > 8F, use manual compression, compression assisted devices, surgical repair, and / or other appropriate treatment methods in the event that bleeding from the femoral access site persists after the use of the Perclose ProGlide™ SMC System to obtain hemostasis.
- For catheterization procedures using a procedural sheath > 8F, where the operating physician is not a vascular surgeon, it is recommended that a vascular surgeon or a surgeon with vascular training be available during the procedure to perform any necessary vascular surgical intervention.
- If the Perclose ProGlide™ Device is used to close and repair multiple access sites in the same vessel, space the access sites apart adequately to minimize sheath-device interference
Potential Adverse Events:
Potential adverse events associated with use of vessel closure devices may include, but are not limited to, the following:
- Allergic reaction or hypersensitivity to device components
- Vascular access complications which may require transfusion or vessel repair, including:
- Anemia
- Aneurysm
- Arteriovenous fistula
- Bleeding / hemorrhage / re-bleeding
- Bruising
- Hematoma
- Embolism
- Inflammation
- Intimal tear / dissection
- Perforation
- Pseudoaneurysm
- Retroperitoneal hematoma / bleeding
- Scar formation
- Wound dehiscence
- Cardiac arrhythmias (including conduction disorders, atrial and ventricular arrhythmias)
- Atrial arrhythmias
- Ventricular arrhythmias
- Femoral artery / venous complications which may require additional intervention, including:
- Arterial / venous stenosis
- Arterial / venous occlusion
- Arteriovenous fistula
- Intimal tear / dissection
- Ischemia distal to closure site
- Nerve injury
- Numbness
- Thrombus formation
- Vascular injury
- Venous thromboembolism (including deep vein thrombosis, pulmonary embolism, post-procedure pulmonary embolism)
- Infection – local or systemic
- Pain
- Hemodynamic instability:
- Hypotension / hypertension
- Vasovagal episode
- Death
- Device complications
- Device failure
- Device malfunction
MAT-2100358 v4.0
StarClose SE™ Vascular Closure System

Indications for Use
The StarClose SE™ Vascular Closure System is indicated for the percutaneous closure of common femoral artery access sites while reducing times to hemostasis, ambulation, and dischargeability in patients who have undergone diagnostic endovascular catheterization procedures utilizing a 5F or 6F procedural sheath.
The StarClose SE™ Vascular Closure System is indicated for use to allow patients who have undergone diagnostic endovascular catheterization procedures to ambulate and be eligible for discharge as soon as possible after device placement.
The StarClose SE™ Vascular Closure System is indicated for the percutaneous closure of common femoral artery access sites while reducing times to hemostasis and ambulation in patients who have undergone interventional endovascular catheterization procedures utilizing a 5F or 6F procedural sheath.
Caution
Federal law restricts this device to sale by or on the order of a physician (or allied healthcare professionals, authorized by, or under the direction of, such physicians) who is trained in diagnostic and therapeutic catheterization procedures and who has been trained by an authorized representative of Abbott Vascular.
Prior to use, the operators must review the Instructions for Use and be familiar with the deployment techniques associated with the use of this device.
Contraindications
The StarClose SE™ Vascular Closure System is contraindicated for use in patients with known hypersensitivity to nickel-titanium.
Warnings
Do not use the StarClose SE™ Vascular Closure System if the packaging or sterile barrier has been previously opened or damaged or if the components appear to be damaged or defective.
DO NOT RESTERILIZE OR REUSE. The StarClose SE™ Vascular Closure System and accessories are intended for single use only.
Do not use the StarClose SE™ Vascular Closure System if the sterile field has been broken where bacterial contamination of the sheath or surrounding tissues may have occurred, since such a broken sterile field may result in infection.
Do not use the StarClose SE™ Vascular Closure System if the puncture site is located above the most inferior border of the inferior epigastric artery (IEA) and / or above the inguinal ligament based upon bony landmarks, since such a puncture site may result in a retroperitoneal hematoma. Perform a femoral angiogram to verify the location of the puncture site.
Do not use the StarClose SE™ Vascular Closure System if the puncture is through the posterior wall or if there are multiple punctures, since such punctures may result in a retroperitoneal hematoma.
Do not use the StarClose SE™ Vascular Closure System if the puncture site is located in the superficial femoral artery or the profunda femoris artery, since such puncture sites may result in a pseudoaneurysm, intimal dissection, or an acute vessel closure (thrombosis of small artery lumen). Perform a femoral angiogram to verify the location of the puncture site.
Precautions
- The StarClose SE™ Vascular Closure System should be used only by operators trained in diagnostic and interventional catheterization procedures who have been certified by an authorized representative of Abbott Vascular Inc.
- The StarClose SE™ Vascular Closure System is provided sterile and non-pyrogenic in unopened undamaged packaging. Products are sterilized with ethylene oxide and intended for single use only. Do not resterilize. Store in a cool, dry place.
- Prior to use, inspect the StarClose SE™ Vascular Closure System to ensure that the sterile packaging has not been damaged during shipment. Examine all components prior to use to verify proper function. Exercise care during device handling to reduce the possibility of accidental device breakage.
- As with all catheter-based procedures, infection is a possibility. Observe sterile technique at all times when using the StarClose SE™ Vascular Closure System. Employ appropriate groin management, as per hospital protocol, post-procedure and post-hospital discharge to prevent infection.
- Use a single wall puncture technique. Do not puncture the posterior wall of the artery.
- Do not use the StarClose SE™ Vascular Closure System to close vessels with diameters less than 5 mm.
- Do not deploy the Clip in areas of calcified plaque.
- The StarClose SE™ Vascular Closure System can be used ONLY with the StarClose Exchange System (included in the StarClose SE™ Vascular Closure System packaging).
- Do not advance or withdraw the StarClose SE™ Vascular Closure Device against resistance until the cause of that resistance has been determined. Excessive force used to advance or torque the StarClose SE™ device should be avoided, as this may lead to significant vessel damage and/or breakage of the device, which may necessitate interventional and/or surgical removal of the device and vessel repair.
MRI Safety Information
The StarClose Clip has been shown to be MR Conditional immediately following implantation. A patient with this implant can be scanned safely immediately after clip placement under the following conditions:
- Static magnetic field of 3 Tesla or less
- Spatial gradient magnetic field of 720 Gauss/cm or less
- Maximum MR system reported whole-body-averaged specific absorption rate (SAR) of 3 W/kg for 15 minutes of scanning.
In non-clinical testing, the StarClose Clip produced a temperature rise of 0.5°C at maximum MR system-reported whole-body-averaged specific absorption rate (SAR) of 3 W/kg for 15 minutes of MR scanning in a 3 Tesla MR system using a transmit/receive body coil.
The MR image quality may be compromised if the area of interest is in the exact same area or relatively close to the position of the StarClose Clip. Therefore, optimization of MR imaging parameters to compensate for the presence of this implant may be necessary.
Adverse Events
Potential adverse events that could be associated with the use of this device include:
- Major Vascular Complications (Composite)
- Vascular Injury Requiring Repair
- Surgery
- Angioplasty
- Ultrasound Guided Compression
- Thrombin Injection or Other Percutaneous Procedure
- New Ipsilateral Lower Extremity Ischemia
- Access Site-related Bleeding Requiring Transfusion
- Access Site-related Infection Requiring Intravenous Antibiotics or Prolonged Hospitalization
- Access Site-related Nerve Injury Requiring Intervention
- Death
- Minor Vascular Complications (Composite)
- Pseudoaneurysm
- Arteriovenous Fistula
- Hematoma (≥ 6 cm)
- Late Access Site-related Bleeding
- Transient Lower Extremity Ischemia
- Ipsilateral Deep Vein Thrombosis
- Transient Access Site-related Nerve Injury
- Access Site-related Vessel Injury
- Access Site Wound-related Dehiscence
- Access Site-related Bleeding Requiring ≥ 30 minutes to Re-achieve Hemostasis
- Localized Access Site Infection Treated with IM or Oral Antibiotics
- UADE
MAT-2114590 v3.0
Prostar™ XL
Percutaneous Vascular Surgical (PVS) System

INDICATIONS FOR USE
The Prostar™ XL PVS System is indicated for the percutaneous delivery of sutures for closing the common femoral artery access site and reducing the time to hemostasis and time to ambulation (patient walks ten feet) of patients who have undergone catheterization procedures using 8.5F to 10F sheaths. (Refer to PRECAUTIONS, SPECIAL PATIENT POPULATIONS sections).
CONTRAINDICATIONS
None known.
WARNINGS
The outer pouch of the Prostar™ XL PVS System and the individual accessories provides the sterile barrier. Do not use the Prostar™ PVS System or accessories if the packaging or sterile barrier have been previously opened or damaged, or if the components appear to be damaged or defective.
DO NOT RESTERILIZE OR REUSE. The Prostar™ XL PVS System and accessories are intended for single use only.
Do not use the Prostar™ XL PVS System if the puncture site is proximal to the inguinal ligament as this may result in a retroperitoneal hematoma.
PRECAUTIONS
- The Prostar™ XL PVS device and accessories should only be used by physicians (or other healthcare professionals authorized by or under the direction of such physicians) after they have been trained in the use of the Prostar™ XL PVS System and accessories, e.g., participation in a Prostar™ XL PVS System training program or equivalent.
- Observe sterile technique at all times when using the Prostar™ XL PVS System. Employ appropriate groin management, as per hospital protocol, post procedure and post hospital discharge to prevent infection.
- Use a single wall puncture technique. Do not puncture the posterior wall of the artery.
- Adequate knot security requires accepted surgical technique as warranted by surgical circumstances and the experience of the operator.
- There are no reaccess restrictions if previous arteriotomy repairs were achieved with an Abbott Vascular Suture Mediated Device.
- Do not insert the Prostar™ XL device into the femoral artery at an angle greater than 45 degrees to the longitudinal plane of the artery.
- Do not advance or withdraw the Prostar™ XL device against resistance until the cause of that resistance has been determined (see CLINICAL PROCEDURE-Device Placement section). Excessive force used to advance or torque the Prostar™ XL device should be avoided as it may lead to significant arterial damage and / or breakage of the device, which may necessitate intervention and / or surgical removal of the device and arterial repair.
- If excessive resistance in advancing the Prostar™ XL device is encountered, withdraw the Prostar™ XL device over a 0.038” (or smaller) guide wire and reinsert the introducer sheath or use conventional compression therapy.
- In the event suture breakage occurs after an initial knot has been tied, care should be taken to avoid excessive force if the reintroduction of the Prostar™ XL device or introducer sheath is required. Any resistance to introduction should result in advancement of an introducer sheath small enough to be introduced without undue force.
- If significant blood flow is evident through or around the barrel of the Prostar™ XL device, do not deploy needles. Remove the Prostar™ XL device over a 0.038” (or smaller) guide wire and insert an appropriately sized introducer sheath.
- Remove the Prostar™ XL sheath before tightening the suture. Failure to remove the sheath prior to tightening the suture may result in detachment of the tip of the sheath.
- Do not attempt to re-deploy Prostar™ XL needles after the needles have been “backed-down” into the sheath (refer to the TECHNIQUE FOR NEEDLE BACK-DOWN section).
- In the event bleeding from the femoral access site persists after the use of the Prostar™ XL device and accessories, use conventional compression therapy.
ADVERSE EVENTS
The following adverse events have been reported and may occur include:
- Device Malfunction
- Device Complication
- Vascular Repair
- Ultrasound Guided Compression
- Transfusion
- Infection Requiring IV Antibiotics
- Hematoma > 6 cm
- AV Fistula
- Nerve Injury
- Pseudoaneurysm
- Deep Vein Thrombosis
- Late Bleeding
- Wound Dehiscence
- Vessel Laceration
- Local Pulse Deficits or Ischemia
- Embolization
- Transitory Local Irritation
- Nerve Injury
- Vascular Spasm
MAT-2114591 v3.0
FemoStop™ Gold Femoral Compression System

Indications For Use
The FemoStop™ Femoral Compression System is indicated for use in the compression of the femoral artery or vein after vessel cannulation and in ultrasound-guided compression repair of a femoral artery pseudoaneurysm.
Contraindications
- Severe peripheral vascular disease due to the risk of arterial thrombosis.
- Critical limb ischemia.
- Overlying skin necrosis and/or infection.
- Arterial injuries above or near the inguinal ligament.
- The inability to adequately compress due to e.g. coexisting very large hematomas, excessive pain or discomfort (despite anesthetics/analgesics).
- Patients not suitable for compression of their femoral artery due to leg edema, femoral nerve compression, or arterial obstruction.
- Femoral artery graft or vein graft due to the risk of damage.
- Ultrasound-guided compression repair of infected femoral pseudoaneurysms.
Warnings
- For one time use only. Do not reuse or resterilize. Do not use if the original sterile package is not intact. Inspect the system carefully prior to use to verify that all parts are present and undamaged.
- Reuse after cleaning attempts, resterilization and repackaging may result in patient/user infections, product deterioration leading to, e.g. reduced concentration of dome pressure, causing bleeding. Do not disassemble or attempt to repair the system.
- Adequate compression may not be obtained in markedly obese patients.
- Avoid releasing pressure suddenly to reduce any risk of flushing thrombotic material into the artery. Release of thrombotic material may result in embolization which could lead to patient injury.
- Do not leave the system on the patient for inappropriately long compressions, as tissue damage may occur. A brief interruption at least every three hours of pressure is recommended during long compression periods. Inappropriately long compression and/or immobilization may increase the risk for thrombosis or embolization which could lead to patient injury or death.
- If arterial/venous hemostasis is not achieved, significant bleeding may occur which could result in patient injury or death.
- While removing the sheath, ensure that the pressure applied is kept low, to avoid damage to the vessel or a “milking” effect. Allowance for slight bleeding at the site is preferred to preclude introduction of thrombus to the vessel.
- To prevent limb ischemia, do not leave artery completely blocked for more than 3 minutes. Check pedal pulse periodically to confirm whether or not flow remains in the vessels.
- To minimize the risk for arterial/venous fistula formation, venous hemostasis should be achieved prior to removal of the arterial sheath.
- Do not apply pressure to a femoral artery stent due to risk of damage.
Precautions
- The FemoStop™ Femoral Compression System should only be used for compression of the femoral artery or vein after vessel cannulation by or on the order of physicians trained in femoral artery or vein compression procedures.
- Federal (USA) law restricts this device to sale by or on the order of a physician.
- When removing the sheath for an Intra-Aortic Balloon Pump (IABP), the Instruction for Use for the IABP should be followed as appropriate.
- For successful compression, the system must be snug and secure around the patient’s hips before pressure is applied. Do not over-tighten the belt.
- For successful compression, the system must be correctly positioned throughout the procedure so that pressure is applied to the point intended.
- On very obese patients, it may be necessary to tighten the belt slightly more to enhance downward compression.
- When using the system on obese patients, fatty tissue may be displaced giving a false impression of a developing hematoma.
- Placement of the system may not be suitable on large patients, or patients with very wide hips as the belt may be too short. An abdominal strap/tape may be used to pull excessive adipose tissue away from dome.
- The target inflation pressure should be 10-20mmHg above the systolic pressure, or higher if necessary to control the bleeding. Exceeding pressures of 200mmHg may indicate the need to tighten the belt or reposition the dome.
- Careful monitoring of the dome pressure during the initial period of use is recommended, as the elastic material of the dome may stretch slightly during the first few minutes. You may notice a slight drop in pressure on the manometer. If this occurs, reinflate to initial pressure.
- Ensure that the control knob on the pump is closed when increasing the pressure and open when decreasing the pressure.
- Ensure that the pinch clamp is open when increasing or decreasing the pressure.
- Use of the FemoStop™ Femoral Compression System is not intended to replace careful monitoring of the patient’s puncture site. The patient should not be left completely unattended during the time of compression.
- The compression system is for single use only.
- Avoid exposing the pump to any liquid.
- After use, this product may be a potential biohazard. Handle and dispose of all such devices in accordance with accepted medical practice and applicable local, state and federal laws and regulations.
- The temperature range on the label represents the temperature for long-term storage.
Adverse Events
Possible adverse effects that may result from the use of this device include but are not limited to:
- tissue necrosis
- blistering of the skin/skin abrasion
- compression injuries to nerves with subsequent sensory and motor deficits
- femoral artery and/or vein thrombosis
- embolization
- bleeding or hematoma
- arterio-venous fistula or pseudoaneurysm
- acute distension or rupture of a pseudoaneurysm during compression repair
Additional warnings and precautions for ultrasound-guided compression repair of a pseudoaneurysm in the femoral artery
Warnings
- To prevent limb ischemia, do not leave artery completely blocked for more than 3 minutes.
- Use color Doppler to periodically confirm whether or not flow remains in the vessels.
- Do not leave the system on the patient for inappropriately long compressions, as tissue damage may occur.
- During long compression periods, pressure should be briefly interrupted at least every three hours. Use manual compression during this break to limit new flow into the pseudoaneurysm.
- Inappropriately long compression and/or immobilization may increase the risk for thrombosis or embolization which could lead to patient injury or death.
Precautions
- The FemoStop™ Femoral Compression System should only be used for ultrasound-guided compression repair of a pseudoaneurysm in the femoral artery, by physicians trained in the treatment of pseudoaneurysm.
- Remove any residual ultrasound gel from the skin overlying the point to be compressed as it may cause the system to slip out of position during the application of pressure.
- Exceeding pressures of 200mmHg may indicate the need to tightly secure the belt or reposition the arch.
MAT-2207286 v2.0