CardioMEMS Implantation Procedure Overview
Implantation of the CardioMEMS PA Sensor
After you have identified your heart failure patient as an appropriate candidate for the CardioMEMS™ HF System, you will schedule them for an implant or refer them to an implanting cardiologist for the procedure.
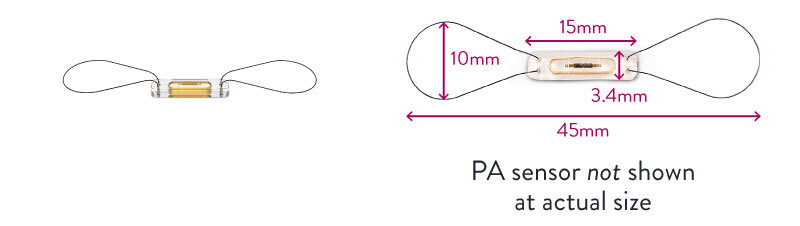
The CardioMEMS™ PA Sensor is optimally inserted into the left descending PA. At the discretion of the implanting physician, the sensor may be inserted into the right PA, depending on the patient’s anatomy. The sensor is about the size of a paper clip when deployed in the target PA. See actual size image and product schematic image below.
Sign up to receive essential information and updates about hemodynamic monitoring with the CardioMEMS HF System
- Clinical Data
- Patient Selection
- Case Studies
- Clinician Education

Watch the CardioMEMS Implant Animation Video
This animation visually explains the implant procedures, but note that each implanting physician will have their own unique procedural technique.
Watch the CardioMEMS Implant Procedure Video
View a live CardioMEMS PA Sensor implant procedure performed by David Shavelle, MD, From Keck School of Medicine USC.
CardioMEMS HF System: Covered by Medicare Advantage*
Get reimbursement resources about expanded coverage for CardioMEMS HF System.
*If coverage criteria is met
MAT-2006909 v7.0