References
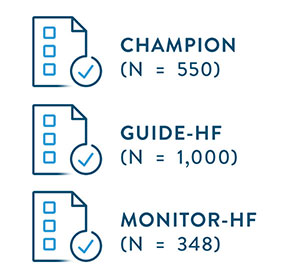
- Lindenfeld J, Zile MR, Desai AS, et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomized controlled trial. The Lancet. 2021;398:991-1001.
- Abraham J, et al. Association of Ambulatory Hemodynamic Monitoring with Clinical Outcomes in a Concurrent Matched Cohort Analysis. JAMA Cardiology. 2019;4(6):556-563.
- Givertz MM, Stevenson LW, Costanzo MR, et al., on behalf of the CHAMPION Trial Investigators. Pulmonary artery pressure–guided management of patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2017; 70:1875–86.
- Abraham WT, Stevenson LW, Bourge RC, et al. (2016). Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: Complete follow-up results from the CHAMPION randomised trial. Lancet, 387(10017), 453-461.
- Adamson et al. Wireless Pulmonary Artery Pressure Monitoring Guides Management to Reduce Decompensation in Heart Failure With Preserved Ejection Fraction. Circulation: Heart Failure. 2014;7;935-944.
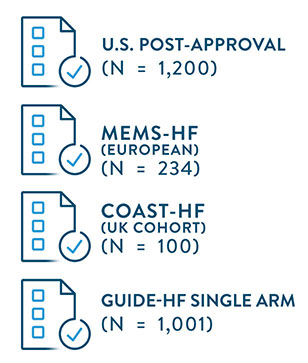
- Shavelle D, et al. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure: One year outcomes from the CardioMEMS Post- Approval Study. Circulation: Heart Failure. 2020; e006836.
- Angermann, C, Aßmus, B, et al. Pulmonary-Artery-Pressure-Guided Therapy in Ambulatory Patients with Symptomatic Heart Failure: The CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF). European J of Heart Failure. 2020. 10.1002/ejhf.1943.
- Heywood JT, Jermyn R, Shavelle D, et al. Impact of practice-based management of PA pressures in 2000 patients implanted with the CardioMEMS sensor. Circulation. 2017; 135: 1509–17.
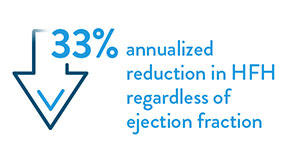
- Desai AS, et al. Ambulatory Hemodynamic Monitoring Reduces Heart Failure Hospitalizations in “Real-World” Clinical Practice. J Am Coll Cardiol. 2017; 69(19):2357–65.
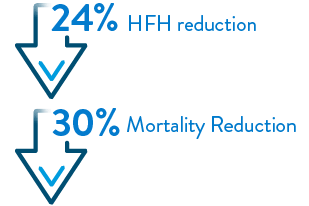
- Lindenfeld, J, Costanzo, M, Zile, M. et al. Implantable Hemodynamic Monitors Improve Survival in Patients With Heart Failure and Reduced Ejection Fraction. J Am Coll Cardiol. 2024 Feb, 83 (6) 682–694.
- Mehra M, Costanzo MR, Zile M, et al; GUIDE-HF Trial Investigators. Primary results of the prospective single arm trial of hemodynamic-guided management of heart failure (GUIDE-HF). Presented at: HFSA Conference; October 2023; Cleveland, OH.
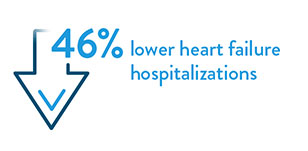
- Brugts, J et al. Remote haemodynamic monitoring of pulmonary artery pressures in patients with chronic heart failure (MONITOR-HF): a randomised clinical trial. The Lancet. May 20, 2023. https://doi.org/10.1016/S0140-6736(23)00923-6.