Clarity in Heart Failure Management: Slow heart failure progression with early intervention using presymptomatic data
Abbott’s CardioMEMS™ HF System is the leading remote monitoring platform for both HFpEF1 and HFrEF patients clinically proven to:
- aid physicians in preventing worsening heart failure2
- lower heart failure mortality rates3-5
- improve quality of life for heart failure patients6
As telemedicine becomes more common, the CardioMEMS HF System continues to be a safe, reliable way to help your patients manage their heart failure.
The CardioMEMS HF System offers you real-time notification of patient changes and simple, convenient access to secure data for proactive, personalized patient management. It also provides patients with a heightened awareness of the factors affecting their health and a powerful sense of control.
CardioMEMS HF System is Covered by Medicare Advantage*
*If coverage criteria is met
Monitoring Pulmonary Artery (PA) Pressures is Proactive and Actionable
The CardioMEMS HF System remotely monitors changes in pulmonary artery (PA) pressure, an early indicator of the onset of worsening heart failure. These early changes can often be addressed through simple adjustments to care, such as titration of medications, most often without requiring an appointment with the patient.
Traditionally, clinicians have focused on physiological markers (patient weight, blood pressure, etc.) to detect worsening heart failure. Unfortunately, these markers appear late in the time course of decompensation. Relying on them leaves little time to respond before hospitalization is necessary.
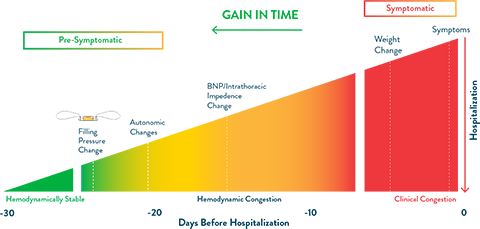
Graph adapted from Adamson PB. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Current Heart Failure Reports. 2009;6:287-292.
Sign up to receive essential information and updates about hemodynamic monitoring with the CardioMems HF System
- Clinical Data
- Patient Selection
- Case Studies
- Clinican Education

The CardioMEMS HF System is the fastest growing technology to manage both HFrEF and HFpEF patients1
The CardioMEMS HF System is proven through clinical trial data to reduce heart failure hospitalizations and mortality,3-5 as well as improve QOL for HFrEF and HFpEF patients.6 The presymptomatic data provided by the CardioMEMS HF System allow for proactive changes in medical therapy before heart failure symptoms appear.6 The ability to individually set patient thresholds allows physicians to personalize and optimize care and medical management for each patient.
References
- Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circulation: Heart Failure. 2014;7(6):935-944.
- Lindenfeld J, Zile MR, Desai AS, et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomized controlled trial. The Lancet. 2021;398:991-1001.
- Abraham J, Bharmi R, Jonsson O, et al. Association of ambulatory hemodynamic monitoring with clinical outcomes in a concurrent matched cohort analysis. JAMA Cardiology. 2019;4(6):556-563.
- Givertz MM, Stevenson LW, Costanzo MR, et al., on behalf of the CHAMPION Trial Investigators. Pulmonary artery pressure–guided management of patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2017;70:1875-86.
- Lindenfeld, J, Costanzo, MR, Zile, MR, on behalf of the GUIDE-HF, CHAMPION, and LAPTOP-HF investigators. Implantable Hemodynamic Monitors Improve Survival in Patients with Heart Failure and Reduced Ejection Fraction. Journal of the American College of Cardiology (JACC). 2024;83(6):682-694.
- Brugts, J et al. Remote haemodynamic monitoring of pulmonary artery pressures in patients with chronic heart failure (MONITOR-HF): a randomised clinical trial. The Lancet. May 20, 2023. https://doi.org/10.1016/S0140-6736(23)00923-6
MAT-2006906 v11.0
Indications, Safety & Warnings
Rx Only
Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events and directions for use.
CardioMEMS™ HF System Indications and Usage: The CardioMEMS HF System is indicated for wirelessly measuring and monitoring pulmonary artery pressure and heart rate in NYHA Class II or III heart failure patients who either have been hospitalized for heart failure in the previous year and/or have elevated natriuretic peptides. The hemodynamic data are used by physicians for heart failure management with the goal of controlling pulmonary artery pressures and reducing heart failure hospitalizations.
CardioMEMS HF System Contraindications: The CardioMEMS HF System is contraindicated for patients with an inability to take dual antiplatelet or anticoagulants for one month post implant.
CardioMEMS HF System Adverse Events: Potential adverse events associated with the implantation procedure include, but are not limited to, the following: air embolism, allergic reaction, infection, delayed wound healing, arrhythmias, bleeding, hemoptysis, hematoma, nausea, cerebrovascular accident, thrombus, cardiovascular injury, myocardial infarction, death, embolism, thermal burn, cardiac perforation, pneumothorax, thoracic duct injury or hemothorax.
MAT-2006766 v7.0