Unmatched 1-Year Patency & 3-Year Freedom From TLR
The Supera™ Stent has been studied in over 2,000 patients worldwide in the SUPERB trial and 16 retrospective studies. Notably, in all of the 17 studies, the Supera™ Peripheral Stent showed durable results with zero fractures at 1 year.1,3-6,9-12,14-21
SUPERB Trial
At 1 year the Supera™ Stent demonstrated primary patency of 91% when nominally* deployed. At 3 years, freedom from targeted lesion revascularization (TLR) was 94% when nominally* deployed.1
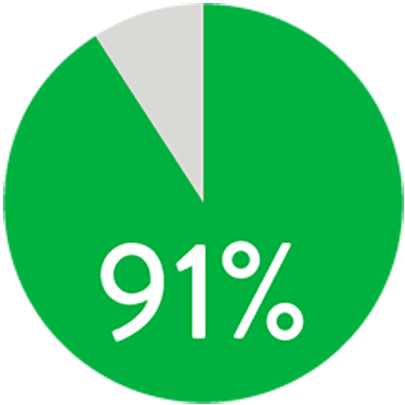
PATENCY (K-M) AT 1 YEAR
When nominally deployed*
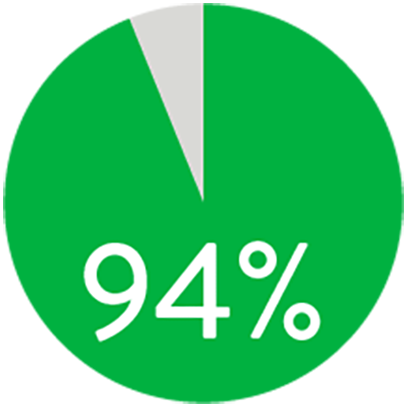
FREEDOM FROM TLR AT 3 YEARS
When nominally deployed*
*Nominal deployment is defined as the stent length upon deployment being within +/- 10% of the labeled stent length. This data is from a non-powered post-hoc analysis. K-M=Kaplan Meier.
Consistent Patency Regardless of Lesion Length
With some peripheral stents, increasing lesion lengths can lead to decreasing patency rates.20 The Supera™ Stent stands apart for its consistently high patency rates in lesions spanning lengths from 5.3 cm up to 28.0 cm*.
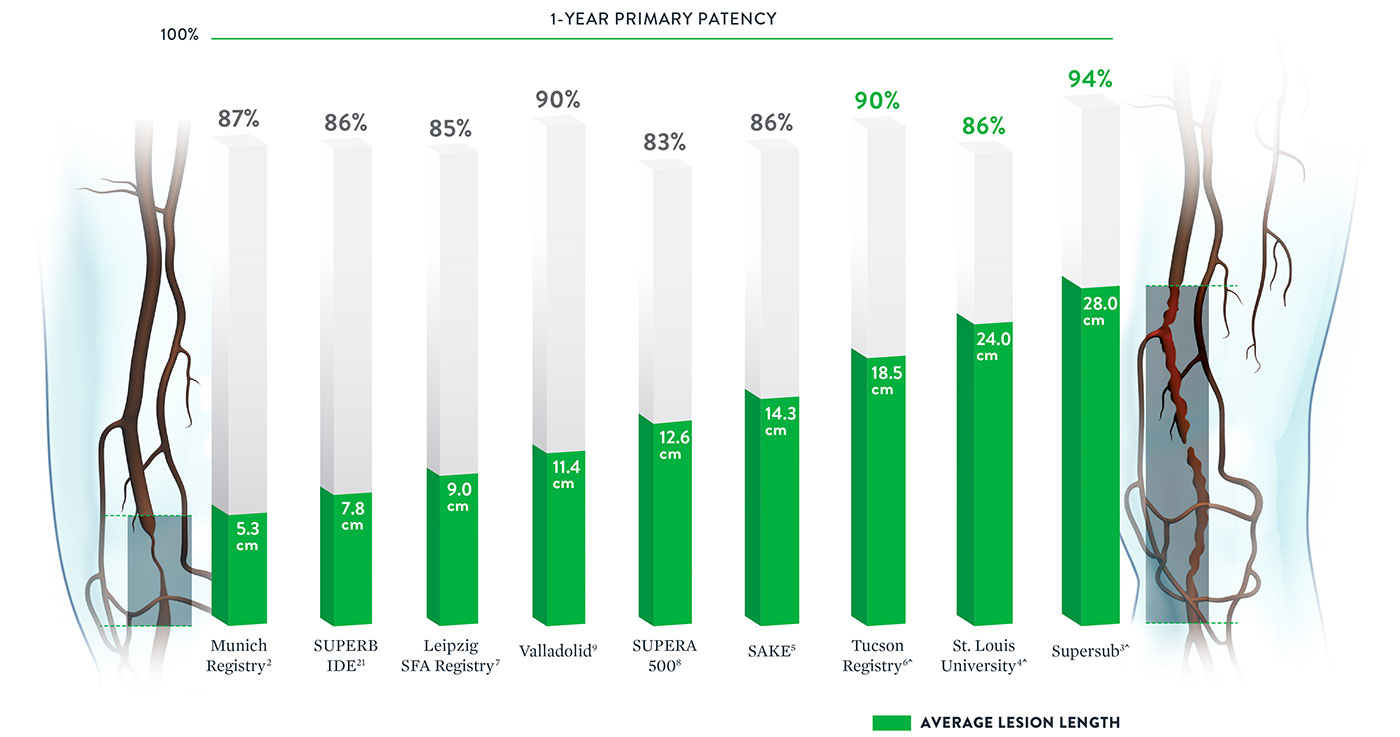
*Published data was included if lesion length and patency were both available. ^Multiple stents used for longer length.
Note: Results from different clinical trials are not directly comparable. Information provided for educational purposes only.
Excellent Patency in Both Simple and Complex Lesions
Whether treating simple (TASC A&B) or complex (TASC C&D) lesions, the Supera™ Stent is associated with impressive, consistent patency performance data.2-4,21
| Simple | |||
|---|---|---|---|
 | Trial/Study | Munich Registry2 | SUPERB21 |
| Lesion Length | 5.3cm | 7.8cm | |
| TASC A&B Lesions | 100% | 94% | |
| 1-Yr Patency | 86.7% | 90.5% | |
| Sites | Single Center | Multicenter (46 sites) | |
| # Patients | 70 | 264 | |
TASC: Trans-Atlantic Inter-Society Consensus
| Complex | |||
|---|---|---|---|
 | Trial/Study | St. Louis4 | SuperSub3 |
| Lesion Length | 5.3cm | 7.8cm | |
| TASC C&D Lesions | 78% | 100% | |
| CTOs | Unknown | 100% | |
| 1-Yr Patency | 85.6% | 94.1% | |
| Sites | Single Center | Single Center | |
| # Patients | 48 | 34 | |
TASC: Trans-Atlantic Inter-Society Consensus
References
- Garcia L. et al., Catheterization and Cardiovascular Interventions 2017 Jun 1;89(7):1259-1267.
- Treitl, K.M., et al. European Radiology.2017; 10.1007.
- Palena L.M. et al. Catheterization and Cardiovascular Intervention.2016.
- Brescia AA. et al., J Vasc Surg. 2015 Jun;61(6):1472-8
- George JC. et al., J Vasc Interv Radiol. 2014 Jun;25(6):954-61.
- Montero-Baker M. et al., J Vasc Surg. 2016 Oct;64(4):1002-8.
- Scheinert D. et al., J Endovasc Ther. 2011 Dec;18(6):745-52.
- Werner M. et al., EuroIntervention. 2014 Nov;10(7):861-8.
- San Norberto EM. et al., Ann Vasc Surg. 2017 May;41:186-195.
- Chan YC. et al., J Vasc Surg. 2015 Nov;62(5):1201-9.
- Dumantepe M. Vasc Endovascular Surg. 2017 Jul;51(5):240-246.
- Goltz JP. et al., J Endovasc Ther. 2012 Jun;19(3):450-6.
- León LR Jr. et al., J Vasc Surg. 2013 Apr;57(4):1014-22.
- Myint M. et al., J Endovasc Ther. 2016 Jun;23(3):433-41.
- Palena LM. et al., J Endovasc Ther. 2018 Oct;25(5):588-591.
- Scheinert D. et al., JACC Cardiovasc Interv. 2013 Jan;6(1):65-71.
- Steiner S. et al., J Endovasc Ther. 2016 Apr;23(2):347-55.
- Teymen B. et al., Vascular. 2018 Feb;26(1):54-61.
- Bhatt H. et al., Cardiovasc Revasc Med. 2018 Jul;19(5 Pt A):512-515.
- Shroë H. Superficial femoral artery PTA or stenting? 5-Year results. CIRSE 2011; Munich, Germany
- Garcia L. et al. Circ Cardiovasc Interv. 2015; 8:e00937.
MAT-2109822 v3.0
Supera™ Peripheral Stent System

Indications
The Supera™ Peripheral Stent System is indicated to improve luminal diameter in the treatment of patients with symptomatic de novo or restenotic native lesions or occlusions of the superficial femoral artery (SFA) and / or proximal popliteal artery with reference vessel diameters of 4.0 to 7.5 mm, and lesion lengths up to 140 mm.
Contraindications
The Supera™ Peripheral Stent System is contraindicated in:
- Patients who are judged to have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the stent or stent delivery system.
- Patients who cannot receive antiplatelet or anticoagulation therapy. Based on in vivo thrombogenicity testing, the device should not be used in patients who cannot be anticoagulated as there may be some thrombus formation in the absence of anticoagulation.
Warnings
- This device is intended for single-use only. Do not reuse. Do not resterilize. Do not use if the package is opened or damaged.
- Use this device prior to the “Use By” date as specified on the device package label. Store in a dry, dark, cool place.
- DO NOT use if it is suspected that the sterility of the device has been compromised.
- Persons with known hypersensitivities to Nitinol and / or its components (e.g. nickel-titanium) may suffer an allergic reaction to this implant.
- Administer appropriate antiplatelet therapy pre- and post-procedure.
- Careful attention should be paid when sizing and deploying the stent to prevent stent elongation. In the SUPERB clinical study, stent elongation was associated with a decrease in patency at 12 months.
Precautions
The Supera™ Peripheral Stent System should only be used by physicians and medical personnel trained in vascular interventional techniques and trained on the use of this device.
- The long-term safety and effectiveness of the Supera™ Peripheral Stent System has not been established beyond three years.
- The safety and effectiveness of the Supera™ Peripheral Stent System has not been established in patients who:
- are less than 18 years old
- are pregnant or lactating
- have in-stent restenosis of the target lesion
- have known hypersensitivity to any component of the stent system (e.g., nickel)
- cannot tolerate contrast media and cannot be pre-treated
- have uncontrolled hypercoagulability and / or another coagulopathy
- This device is not designed for use with contrast media injection systems or power injection systems.
- The flexible design of the Supera™ stent may result in variation in the deployed stent length.
Magnetic Resonance Imaging (MRI) Safety Information
Nonclinical testing has demonstrated that the Supera™ stent, in single and in overlapped configurations up to 250 mm in length, is MR Conditional. A patient with this device can be safely scanned in an MR system meeting the following conditions:
- Static magnetic field of 1.5 or 3.0 Tesla
- Maximum spatial gradient magnetic field of 2,500 Gauss/cm (25 T/m)
- Maximum MR whole-body-averaged specific absorption rate (SAR) of
- 2 W/kg for landmarks (i.e. center of RF coil) above the umbilicus
- 1 W/kg for landmarks below the umbilicus and above the mid-thigh
- 0.5 W/kg for landmarks below the mid-thigh
Under the scan conditions defined above, the Supera™ stent is expected to produce a maximum temperature rise of 7.6 °C after 15 minutes of continuous scanning.
In nonclinical testing, the image artifact caused by the device extends approximately 2 cm from the Supera™ stent when imaged with a gradient echo or spin echo sequence and a 3T MRI system.
Potential Adverse Events
Potential adverse events include, but are not limited to:
- Abrupt closure
- Allergic reaction (contrast medium; drug; stent material)
- Amputation or limb loss
- Aneurysm or pseudoaneurysm in vessel or at vascular access site
- Angina or coronary ischemia
- Arrhythmia (including premature beats, bradycardia, atrial or ventricular tachycardia, atrial or ventricular fibrillation)
- Arteriovenous fistula
- Bleeding complications requiring transfusion or surgical intervention
- Death
- Detachment of a system component or implantation in an unintended site
- Embolization, arterial or other (e.g. air, tissue, plaque, thrombotic material, or stent)
- Emergent surgery
- Fever
- Hematoma or hemorrhagic event, with or without surgical repair
- Hyperperfusion syndrome
- Hypertension / Hypotension
- Infection
- Myocardial infarction
- Pain (leg, foot, and/or insertion site)
- Partial stent deployment
- Peripheral nerve injury
- Pulmonary embolism
- Renal failure or insufficiency
- Restenosis of vessel in stented segment
- Shock
- Stent malapposition or migration, which may require emergency surgery to remove stent
- Stent strut fracture
- Thrombosis or occlusion
- Stroke
- Transient ischemic attack
- Venous thromboembolism
- Vessel dissection, perforation or rupture
- Vessel spasm or recoil
- Worsening claudication or rest pain
MAT-2103597 v3.0
