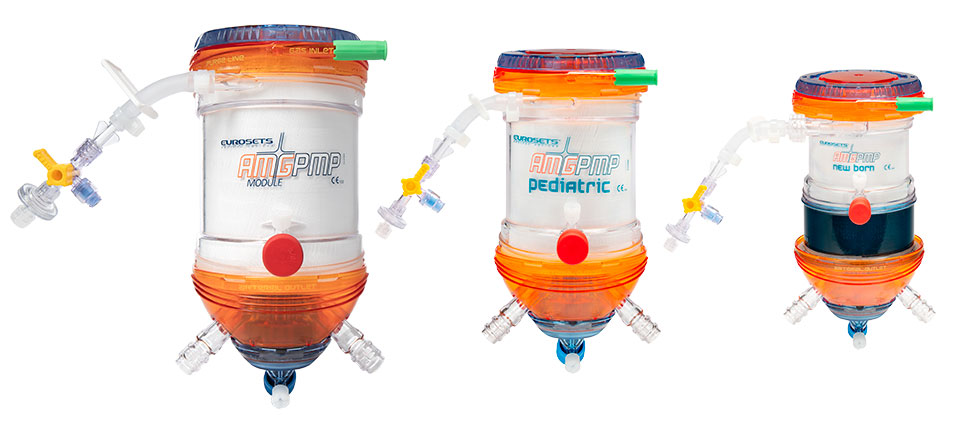
AMG PMP Oxygenators
Manufactured by Eurosets and distributed by Abbott, the AMG PMP Oxygenators easily integrate with the CentriMag™ Acute Circulatory Support System.
AMG PMP Oxygenator benefits and features:
- PMP fibers – for reliable gas exchange performance over duration of use
- PC Coating – to minimize inflammation1 and thrombosis2
- Stainless steel heat exchanger – can eliminate risk of any potential contamination
- Low shear stress – based on shape, fiber orientation, and blood flow path
| Technical Specifications | |||
|---|---|---|---|
| AMG PMP Adult Oxygenator | AMG PMP Pediatric Oxygenator | AMG PMP Infant Oxygenator | |
| Maximum Blood Flow Rate | 7 L/min | 4 L/min | 1.5 L/min |
| Priming Volume | 220 mL | 190 mL | 90 mL |
| Residual Blood Volume | < 200 mL | 100 mL | 41 mL |
| Membrane Surface Area | 1.81 m² | 1.35m² | 0.69m² |
| Heat Exchanger Surface Area | 0.08 m² | 0.08m² | 0.04m² |
| Heat Exchanger Performance Factor | 0.64 at 4 L/min | 0.64 at 4 L/min | 0.77 at 1.5 L/min |
| Max Gas Pressure | 0.14 PSI (7 mmHg) | 0.14 PSI (7 mmHg) | 0.07 PSI (3.5 mmHg) |
| Venous Inlet and Arterial Outlet | 3/8" barbed connectors | 3/8" barbed connectors | 1/4" barbed connectors |
| Membrane Material | PMP with PC coating | ||
| Heat Exchanger Type | Stainless steel | ||
| Max Water Pressure | 29 PSI (1,500 mmHg) | ||
| Max Blood Pressure | 14.5 PSI (750 mmHg) | ||
| Water Inlet and Outlet | 1/2" Hansen‡ quick couplings | ||
| Gas Inlet | 1/4" | ||
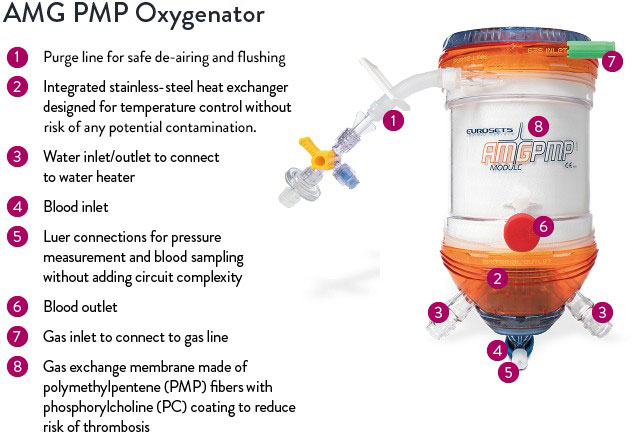
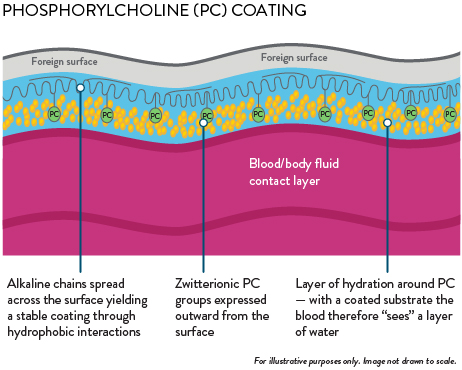
Blood path fully coated with phosphorylcholine (PC) coating
PC creates a permanent water barrier between blood and the oxygenator surface that minimizes thrombus formation and promotes resistance to bacterial adhesion.3
Integrated heat-exchanger maximizes heat exchange efficiency
The integrated heat exchanger is:
- Made of stainless steel for high heat conductivity
- Non-porous to eliminate risk of bacterial infection or any other potential contamination
- Corrugated to increase surface area for efficient heat exchange

AMG PMP Adult Oxygenator
Watch this video to see the technical features of the AMG PMP Adult Oxygenator.
CentriMag System Animation
Watch this video featuring the CentriMag System and the AMG PMP Adult Oxygenator as a BETTER TOGETHER option for cardiopulmonary support.
AMG PMP Oxygenator and Oxygenator Holder manufactured by Eurosets s.r.l., and distributed by Abbott.
ECMO clearance for >6-hour use is indicated for the CentriMag™ Blood Pump to be used with: CentriMag™ Console, CentriMag™ Motor, Mag Monitor, and Flow Probe. ECMO clearance for the CentriMag™ Blood Pump is for adult use only and excludes: CentriMag™ Drainage Cannula and CentriMag™ Return Cannula, PediMag™ Blood Pump and any other pediatric components or accessories.
References
- Hayward JD, Champ D. Biomembrane surfaces as models for polymer design: the potential for haemocompatibility. Biomaterials. 1984;5:135-142.
- Goreish HH, Lewis Al, Rose S, et al. The effect of phosphorylcholine-coated materials on the inflammatory response and fibrous capsule formation: In vitro and in vivo observations. J Biomed Mater Res. 2004;68A:1-9.
- Berry JA, Biedlingmaier JF and Whelan PH. In Vitro Resistance to Bacterial Biofilm Formation on Coated Fluroplastic Tympanostomy Tubes. Otolaryngol Head Neck Surg. 2000;123:246-51.
MAT-2207130 v5.0

Stay Connected