AVEIR AR Atrial LP. AVEIR VR Ventricular LP. Individually, they are capable of AAI(R) or VVI(R). But together they can provide the world's first leadless DDD(R) system. Tailor therapy by implanting one or both devices based on your patient’s indications and needs.
Learn more about the benefits of this system that is redefining the landscape of cardiac pacing below.
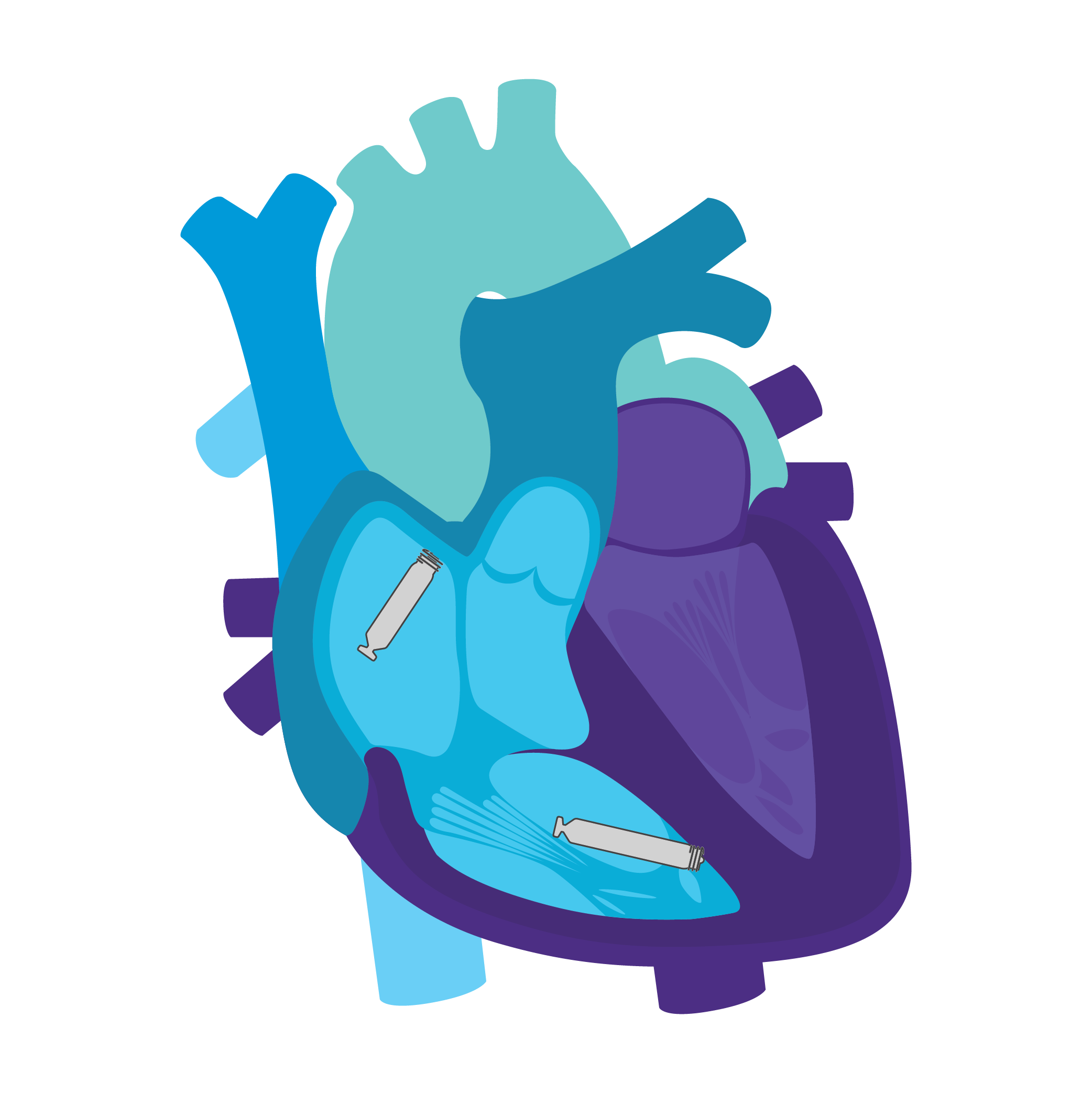
AVEIR™ DR DUAL CHAMBER LP SYSTEM
Leadless pacing has been limited to the right ventricle, necessitating the use of conventional pacemakers. Now, patients can experience the power of atrial only leadless pacing with an AVEIR AR LP. AVEIR AR LP offers a safe3, effective3, and upgradeable1 pacing solution for patients with sinus node dysfunction and normal AV and intraventricular
conduction systems.
ELECTRICAL PERFORMANCE SUCCESS RATE3 in early clinical evidence patients with acceptable atrial device capture threshold and sensing amplitude.*
FREEDOM FROM COMPLICATIONS.3 The inclusion of arrhythmias as a safety end point increased the overall incidence of complications as compared with other studies of leadless pacemakers, which excluded arrhythmias from the end point.
*Atrial device capture threshold of ≤3.0V@04ms and ≥0.5mV at 3 month visit.
Patient therapy can be tailored by implanting an atrial or ventricular device alone, or both combined for dual chamber support. The option to upgrade over time allows you to meet your patient’s needs today and adapt to common disease progression later.
Treatment option 1: Start with the atrial device.
Treat sinus node dysfunction today.
Add a ventricular device for heart block later.
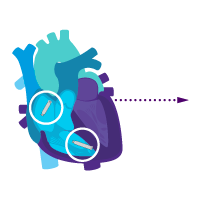
i2i™ communication is enabled.

Activate dual chamber pacing therapy DDD(R) via i2i communication.
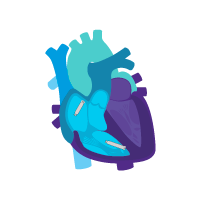
Treatment option 2: Start with the ventricular device.
Treat rare intermittent heart block today.
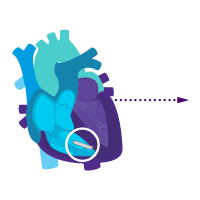
Add an atrial device for sick sinus syndrome later.

i2i communication is enabled.

Activate dual chamber pacing therapy DDD(R) via i2i communication.

The AVEIR VR LP's predicate device has an overall long-term retrieval success rate above 88% with helix fixation through 9 years of retrieval experience.5 Limited data is available for AVEIR VR LP.
The AVEIR VR LP's predicate device has an overall long-term retrieval success rate of 92.5% for implants ≥ 5 years.5
Increased projected battery longevity over current available leadless pacemakers4,6* opens the door to more patients.
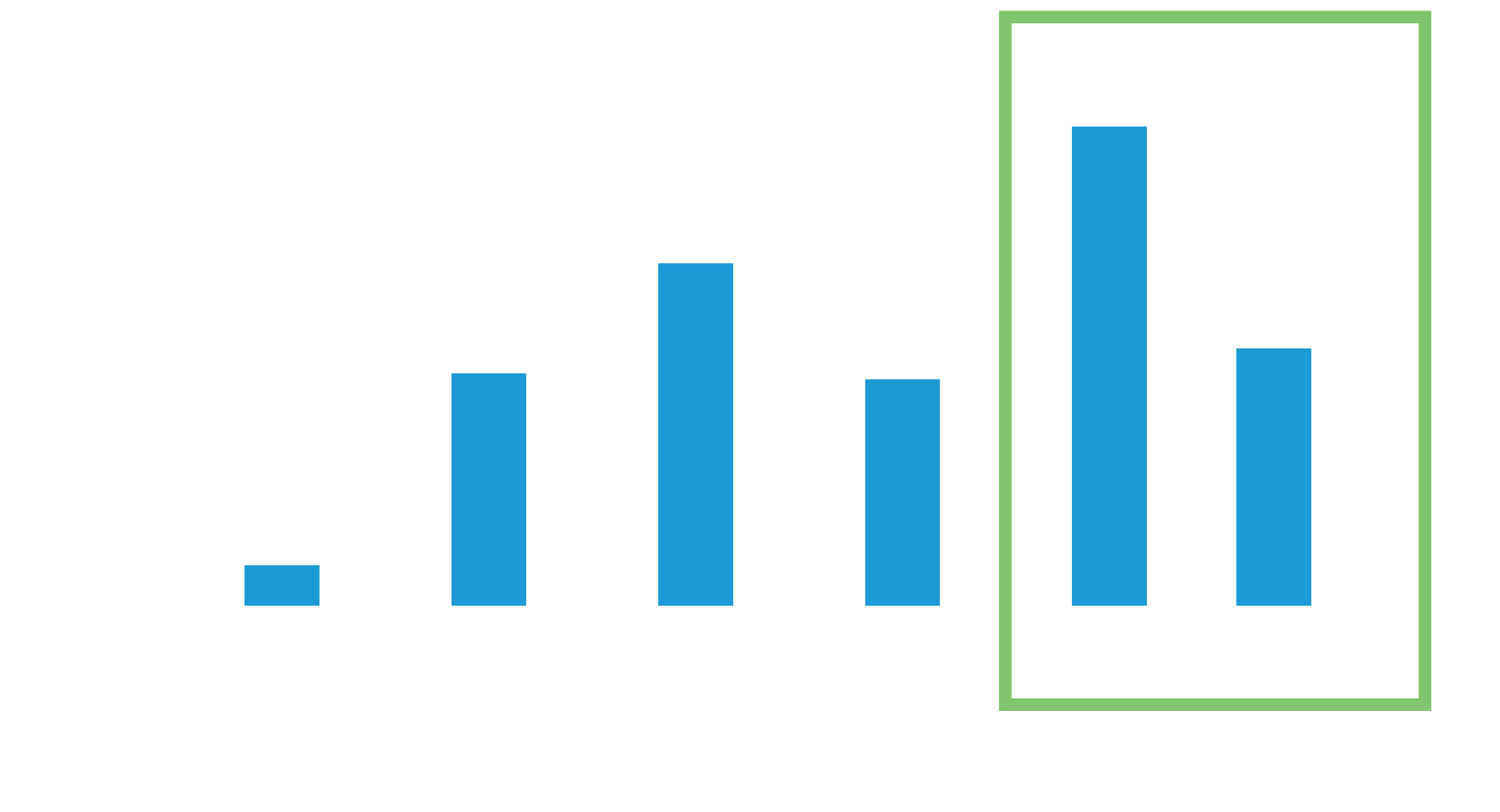
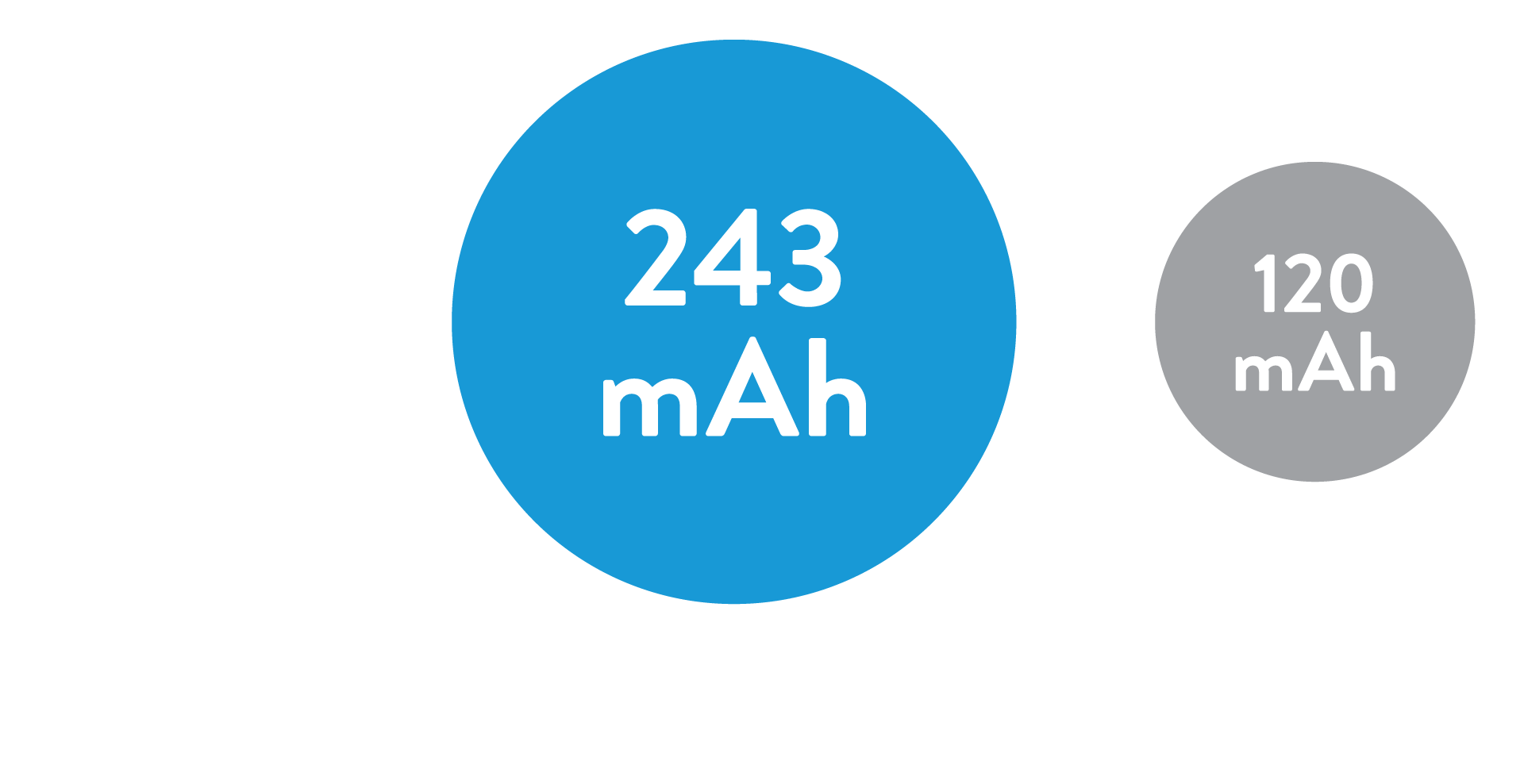
The average battery longevity among Leadless II phase 2 IDE patients at 1 year follow-up is estimated to be 17.6 years7
48% of the study patients have an estimated battery longevity of over 20 years.7
REFERENCES
* Battery longevity estimates based on projections derived from published technical specifications and the ISO standard settings
**For additional information about specific MR Conditional, including warnings, precautions, adverse conditions to MRI scanning and potential adverse events, please refer to the MRI-Ready Leadless Systems Manual at medical.abbott/manuals or check our MRI-Ready resources at cardiovascular.abbott/mriready
ADDITIONAL REFERENCES
MAT-2115643 v10.0 | Item is approved for U.S. use only
Stay Connected