MAT-2514762 v1.0 | Item approved for U.S. use only. ©2025 Abbott. All Rights Reserved.
Minimally invasive. Catheter based.
The World's First Dual Chamber Leadless Pacemaker System1 does not require chest incisions or pockets.
Our innovative leadless technology allows both atrial and ventricular devices to be implanted in the heart through a minimally invasive catheter procedure. A protective sleeve fully covers the leadless pacemaker during catheter navigation to reduce the risk of device damage and injury to cardiovascular structures.
AVEIR™ DR Leadless Pacemaker System Implant Procedure2
PREP the patient's skin and apply EKG electrodes. Connect to the Merlin™ Patient Care System (PCS) Programmer via the EKG cable and AVEIR Link Module.
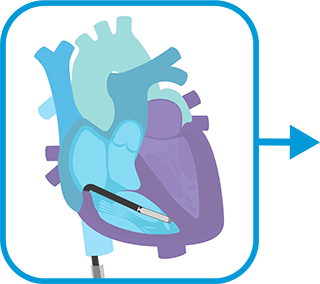
After gaining femoral vein access and visualizing the heart's anatomy and size via imaging, INSERT delivery catheter with attached ventricular leadless pacemaker. Navigate to right ventricle and map for ideal implant site.
FIXATE ventricular leadless pacemaker, go into tether mode to assess electrical measurements, release, and then withdraw the delivery catheter.
Load the atrial leadless pacemaker onto a new delivery catheter, INSERT and navigate to right atrium and map for ideal implant site.
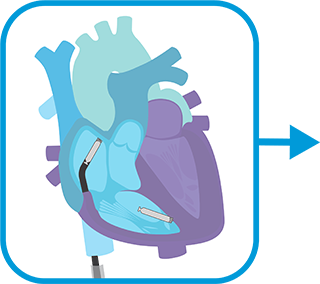
FIXATE atrial leadless pacemaker, go into tether mode to assess electrical measurements, finalize atrial device.
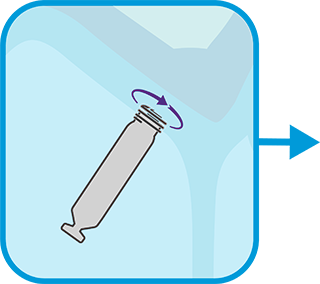
PAIR devices via i2i™ technology, assess i2i™ communication between the two pacemakers, and program dual chamber or an appropriate mode.
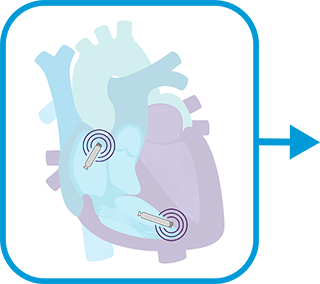
RELEASE atrial device, remove delivery catheter and introducer and close access.
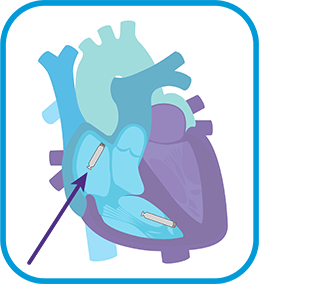
For a more detailed workflow, refer to the AVEIR™ DR Instructions For Use (IFU) or contact your Abbott Sales Representative for training.
Innovative Delivery & Retrieval Catheter Technology2,3,4
- Ergonomic, single operator use.
- Steerable catheters with deflection mechanism.
- Protective sleeve fully covers LP's helix to reduce risk of damage and injury.
- Hydrophilic coating on introducer sheaths with 30 and 50 cm lengths.
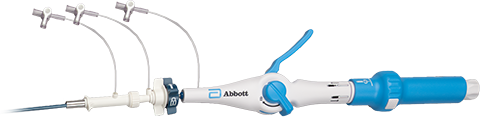

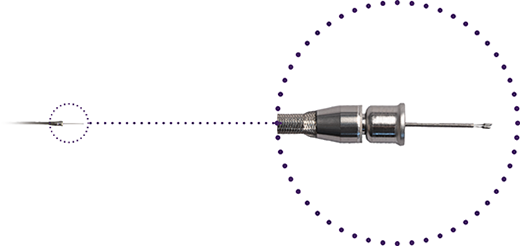
Delivery Catheter
Misaligning and aligning the ends of the tethers allows for the loading and release of the pacemaker.
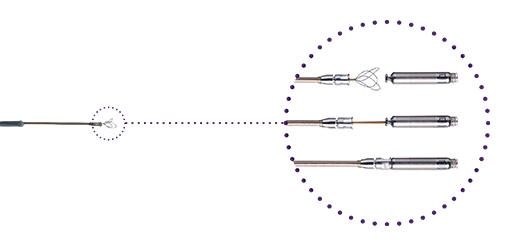
Retrieval Tri-Loop Snare
Re-docking mechanism enables retrieval of the pacemaker.
Enabling Safe MRI Scans
AVEIR™ leadless pacemakers are MR Conditional for full body scans using a 1.5T or 3T field strength MRI scanner.
Stay Informed
Sign up to hear about our technology, education opportunities, and more.
Read the Latest Blog Article
Stay up to date with recent news, product highlights, and case studies.
Intended for U.S. residents only. Please review our privacy policy and terms & conditions.
References
- AVEIR™ DR FDA Approval.
- AVEIR™ Leadless Pacemakers and Delivery Catheter IFU, ARTEN600284235.
- AVEIR™ Retrieval Catheter IFU. ARTMT600174816
- AVEIR™ Introducer IFU. ARTMT600174817
MAT-2307565 v3.0