MAT-2602106 v1.0 | Item approved for U.S. use only. ©2026 Abbott. All Rights Reserved.
Introducing the World's First and Only Dual Chamber Leadless Pacemaker System
Brought to you only by Abbott, the AVEIR DR Dual Chamber Leadless Pacemaker (LP) System is first-of-its-kind technology that provides:
- Beat-to-Beat Synchrony between both chambers.1,2
- Upgradeable from single to dual chamber therapy.1
- Long-Term Retrieval to remove hardware at end of service (EOS).1,3
- Electrical Mapping Before Fixation to reduce repositioning.1,2
Our life-changing technology provides all the clinical benefits of traditional dual chamber pacemakers without the downsides. This means:
- NO pocket- and lead-related complications (including infection, lead fracture, insulation problems, skin erosion and keloid formation).
- NO visible scarring.
- NO arm movement restrictions post-implantation.
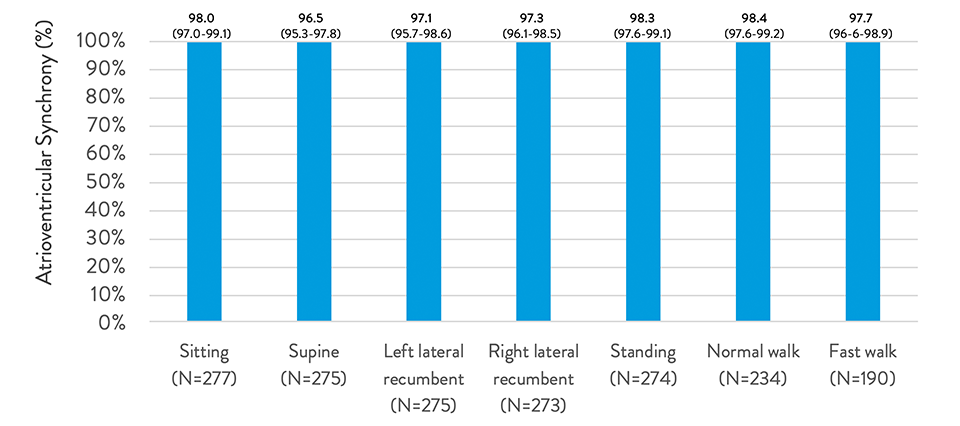
Beat-to-Beat Synchrony1,2
AVEIR™ DR Leadless Pacemaker System is capable of pacing and sensing in both chambers through the combination of an atrial leadless pacemaker (AVEIR™ AR LP) and a ventricular leadless pacemaker (AVEIR™ VR LP).
Dual chamber, leadless synchronous pacing between the atrium and the ventricle is made possible with industry-first, proprietary i2i™ communication technology capable of providing true DDD(R) pacing for continuous, atrioventricular (AV) synchrony, regardless of patient posture.

Implant-to-Implant (i2i™) Technology1
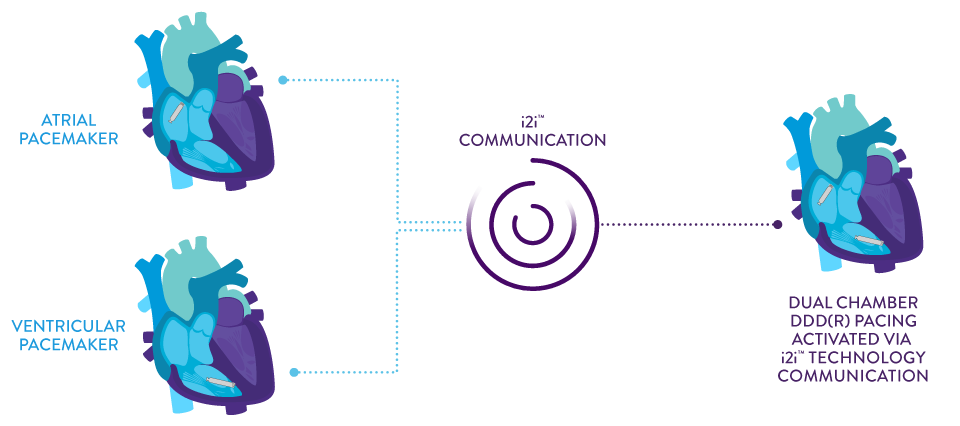
This novel technology features paired, co-implanted devices employing low-energy, subthreshold pulses between them using the conductive nature of the body’s blood pool and myocardial tissue.
High-frequency pulses are delivered concurrently with each locally paced or sensed event without impact on pacing or intrinsic sensing.
Upgradeable System1
Patient therapy can be tailored by implanting an atrial or ventricular device alone, or both combined, for dual chamber support. The option to upgrade over time allows you to:
- Meet your patients’ needs today
- Adapt to common disease progression to meet your patients’ needs in the future



Option to Start with Atrial Pacing
Treat sinus node dysfunction today.
Add Ventricular Pacing Later
Treat those same patients by adding a ventricular device if heart block develops later to provide DDD(R) or AAI(R)+VVI therapy
Achieve Dual Chamber Pacing
Now you have options to adapt to patient needs over time.
Tailored Therapy for Your Leadless Patients
The AAI(R) + VVI Pacing Mode, available for new and existing sinus node dysfunction patients with intact AV conduction, provides increased atrial and ventricular device longevities with beat-to-beat communications turned off and offers primary atrial pacing support with ventricular back-up.4
Up To 70% Increased Atrial Device Longevity
with AAI(R) + VVI Pacing Mode
With beat-to-beat communications turned off, up to 70% increase in projected atrial device longevity compared to DDD(R).*,1

Long-term Retrieval1,3,5
This allows for the replacement of the atrial or ventricular device at end of service (EOS) without leaving hardware behind.
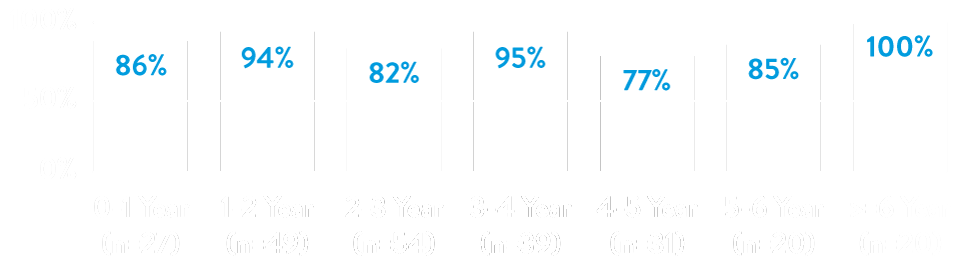
The AVEIR™ VR Ventricular Leadless Pacemaker predecessor has a long-term retrieval success rate above 88% with helix fixation through 9 years regardless of implant duration.3
Retrieval Success by Implant Duration3
The specially designed retrieval catheter provides added confidence and is supported by a step-by-step retrieval protocol.5 AVEIR™ Leadless Pacemakers have an active fixation design that uses a screw-in mechanism to enable both implantation and long-term retrieval of the atrial and ventricular devices.1
Electrical Mapping Prior
to Fixation1,2
Electrical mapping prior to fixation is designed to help reduce the number of repositioning attempts in the atrium and ventricle. AVEIR™ Leadless Pacemakers can assess Current of Injury via Commanded EGM, initial pacing capture thresholds, sensing amplitudes, and impedance in both atrial and ventricular chambers.
of patients had successful VENTRICULAR IMPLANTS WITH 1 OR LESS REPOSITIONING ATTEMPTS.2
of patients had successful ATRIAL IMPLANTS WITH 1 OR LESS REPOSITIONING ATTEMPTS.2
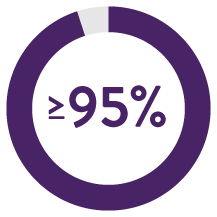
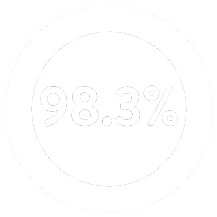
Proven Clinical Evidence2
Across 300 attempted implants, the procedure was successful in 295 patients for a
Dual Chamber Procedure Success Rate2
View safety and efficacy results observed at the three-month follow-up of the AVEIR™ DR i2i™ Study as cited in the New England Journal of Medicine.
Setting the Pace with Dual Chamber Leadless Pacing
Stay Informed
Sign up to hear about our technology, education opportunities, and more.
Read the Latest Blog Article
Stay up to date with recent news, product highlights, and case studies.
Intended for U.S. residents only. Please review our privacy policy and terms & conditions.
References
*1.5V, 0.4ms, 60bpm, 300 ohms, 100% A pacing, 10% V pacing, i2i™ settings = 4 / 4.
- AVEIR™ Leadless Pacemakers and Delivery Catheter IFU, ARTEN600284235.
- Knops, Reinoud E., et al. "A Dual-Chamber Leadless Pacemaker." New England Journal of Medicine (2023). DOI: 10.1056/NEJMoa2300080
- Reddy, VY, et al. Worldwide Experience with Leadless Pacemaker Retrievals: A Worldwide Nanostim Experience out of 9y. Presented at: APHRS 2022; Nov 18-20, 2022; Singapore.
- AVEIR™ Leadless Pacemakers and Delivery Catheter IFU, ARTEN600331825. 2. Abbott. Data on file, Supplemental Longevity Assessments of AVEIR DR System. Item: 91070507.
- AVEIR™ Retrieval Catheter IFU. ARTMT600174816.
MAT-2307559 v6.0