Go Beyond the Limitations of Angiography with OCT Imaging
Ultreon™ 1.0 Software is an intravascular imaging and coronary physiology software on one platform to guide percutaneous coronary intervention (PCI). Ultreon™ 1.0 Software is developed for Optical Coherence Tomography (OCT), used with OPTIS™ Next Imaging Systems.
Streamlined and intuitive, Ultreon™ 1.0 Software gives better insights to optimize patient outcomes through automation and an improved workflow.1-4
Performing PCI based on coronary angiography alone is inadequate for determining key metrics of the vessel such as the extent of coronary artery disease, plaque distribution and composition, and dimensions.5 Ultreon™ Software for OCT allows interventional cardiologists to see inside the heart during PCI and act on the obtained insights.
OCT is an imaging modality that uses near-infrared light to provide high-definition images of the artery with high precision allowing for the assessment of lesion characteristics and plaque morphology of coronary artery disease.
- Ultreon™ Software helps to guide physicians through PCI step-by-step following MLD MAX workflow and provides insights on morphology, vessel sizing, stent placement and post-stent optimization for more accurate decision-making.6
- Ultreon™ Software is powered by artificial intelligence (AI) that enables automatic quantification of calcification and vessel sizing.1
Experience the Power of Automation
With the Ultreon™ 1.0 Software Simulation
- Explore the software's intuitive interface
- Review the streamlined workflow
- Interact with AI-powered auto-detection and measurement features
- Step through image acquisition for OCT and physiology


Ultreon™ 1.0 Software for OCT Imaging was named a CES 2022 Innovation Award Honoree for outstanding design and engineering.

See Simply & Act Decisively During PCI with Ultreon™ 1.0 Software
See Simply
The Ultreon™ Software with an intuitive interface offers physicians user-friendly on-screen information and step-by-step guidance following MLD MAX workflow to aid with decision-making,6 to determine a proper treatment technique pre-PCI and to ensure optimal stent expansion results post-PCI.
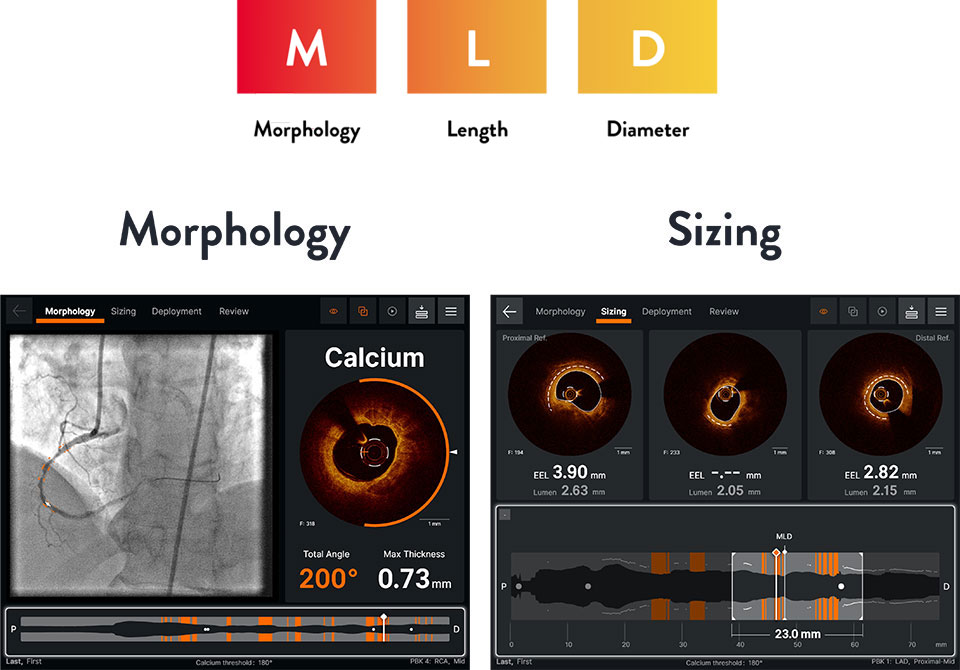
Ultreon™ 1.0 Software intuitive workflow interface - MLD
Ultreon™ 1.0 Software intuitive workflow interface - MAX
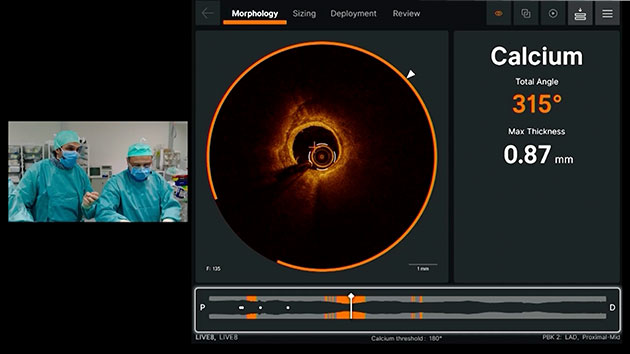
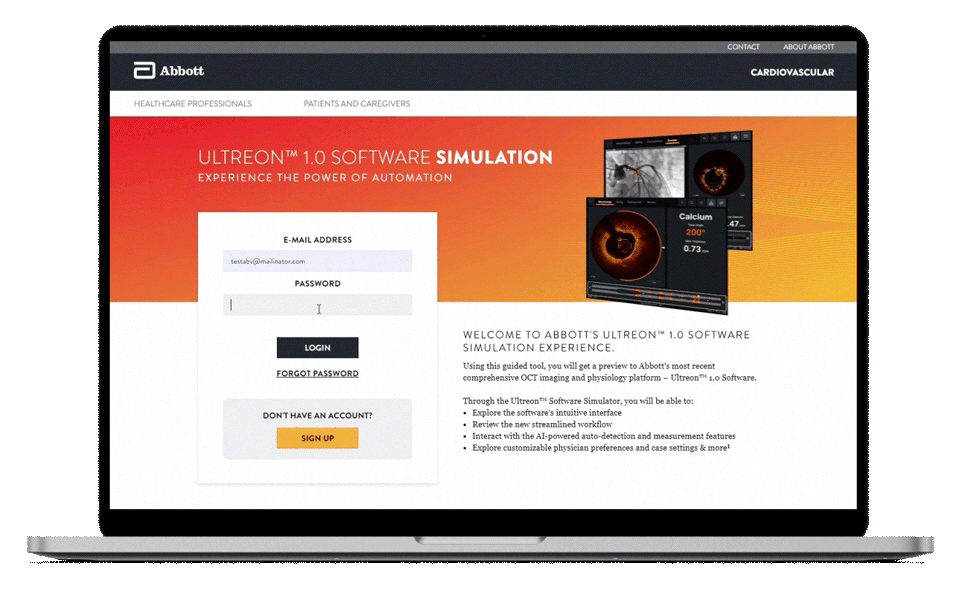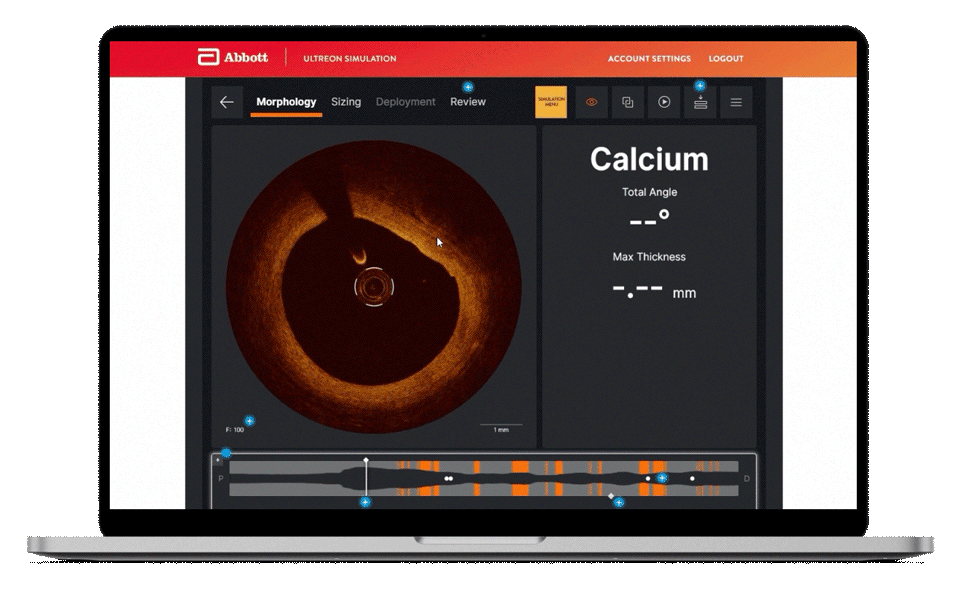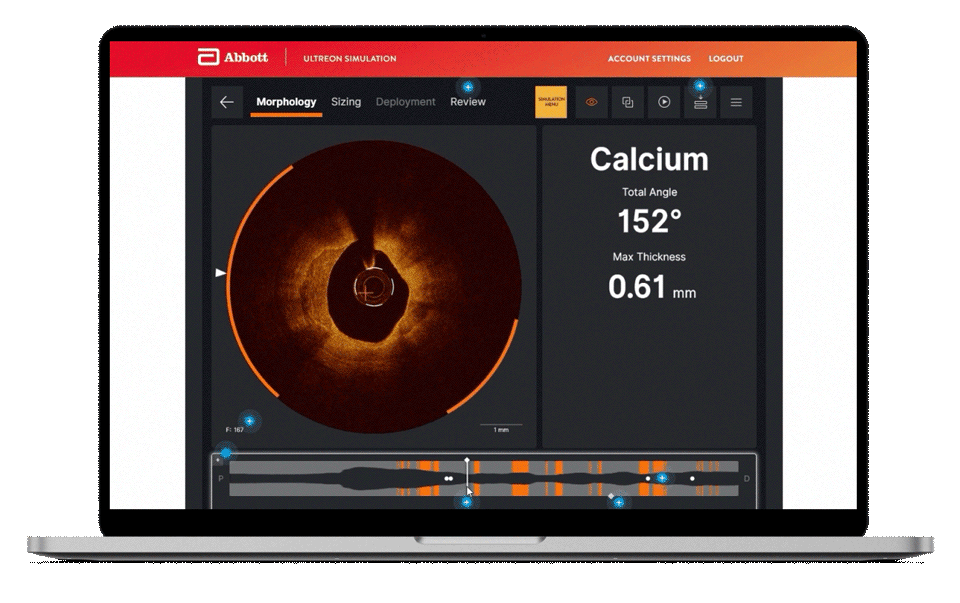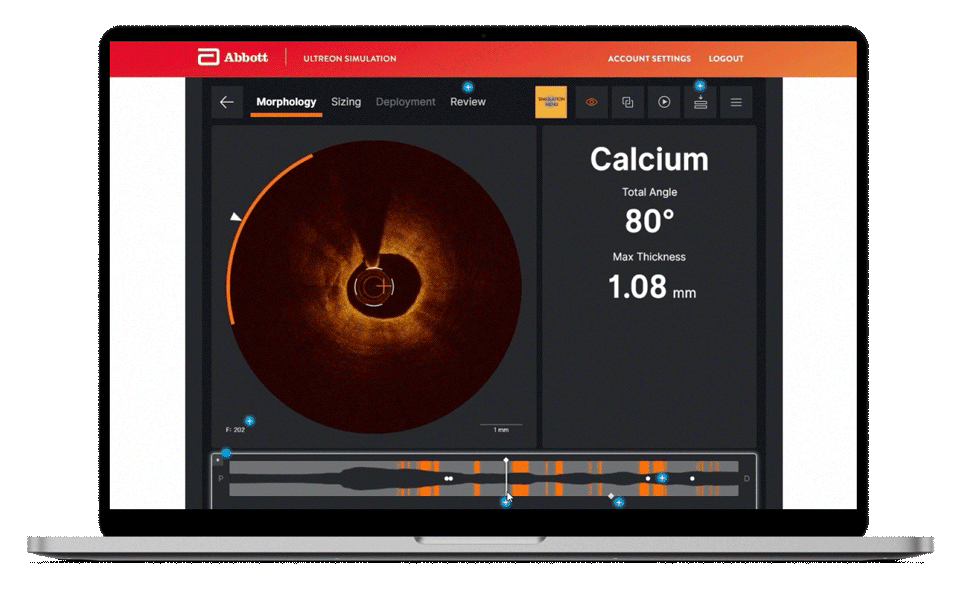
Morphology
Ultreon™ 1.0 Software uses AI to automatically detect degree and thickness of calcification.1
- Auto-detects and highlights calcification arc and max thickness7
- Displays calcification angle and max thickness values throughout the pullback in real-time7
- Highlights calcification overlays on the vessel with the co-registration view7
Watch how to use Ultreon™ 1.0 Software for morphology assessment
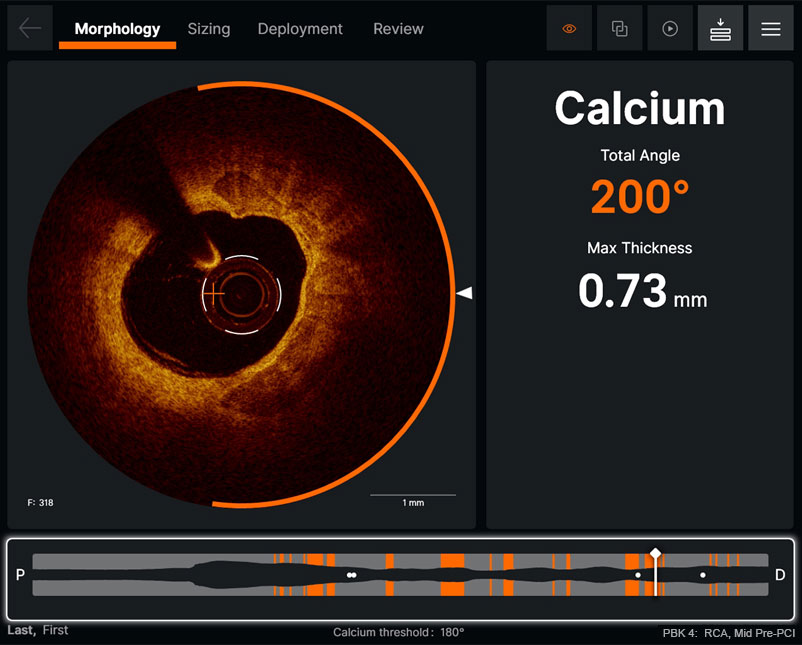
Morphology screen displaying angle and max thickness of calcification
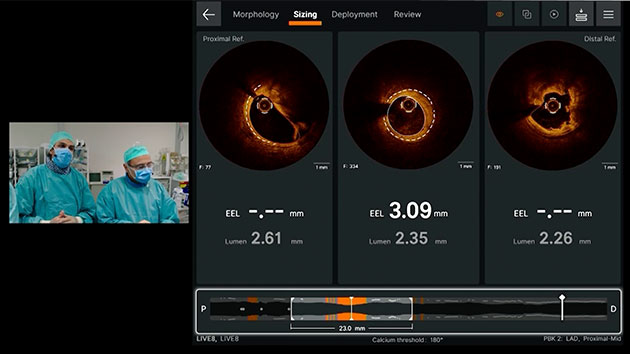
Sizing
Ultreon™ 1.0 Software uses AI to automatically detect external elastic lamina (EEL) and lumen to help identify landing zones for accurate stent placement.1,8
- Provides vessel diameter measurements7
- Facilitates measuring lesion length and identification of optimal user-adjusted stent landing zones on a single screen7
- Autodetects and provides measurement of lumen and detectable EEL for quick visualization7
- Facilitates stent length selection through co-registration7
Watch how to use Ultreon™ 1.0 Software to determine stent sizing
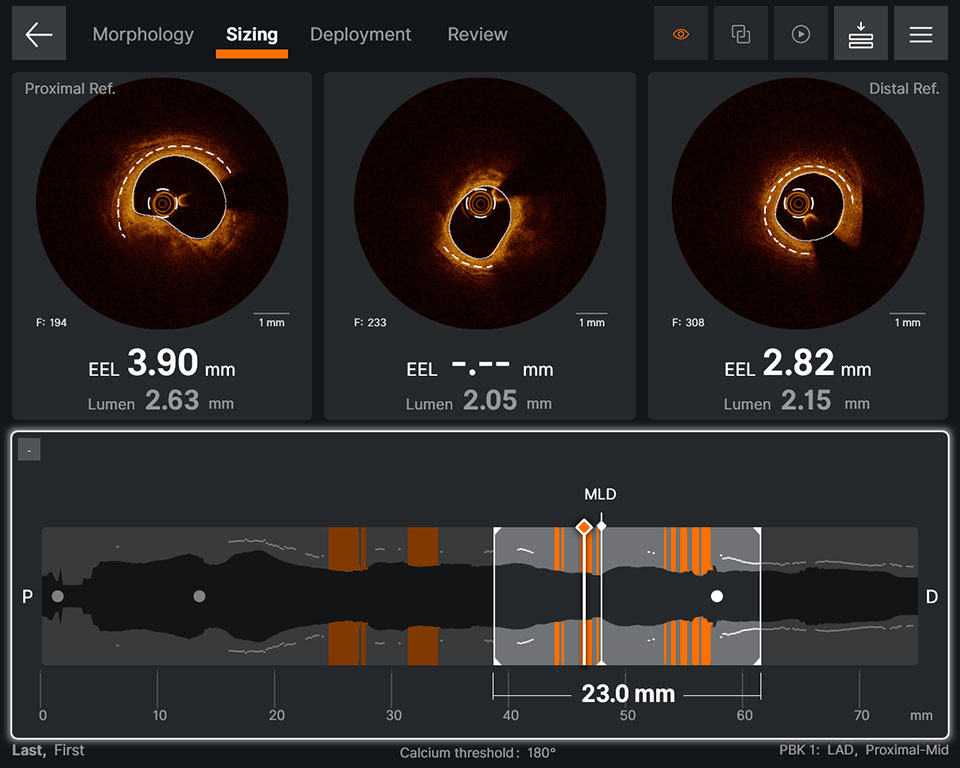
Sizing screen displaying
- EEL: proximal and distal frames
- Lumen diameter: proximal, current and distal frames
"I do OCT in 95% of all my cases and the data-driven guided interface with Ultreon™ Software has ignited an imaging revolution, making imaging interpretation easier (through automatic EEL and calcium detection) and the supported guided-user interface."
– Dr. Jonathan Hill, London UK

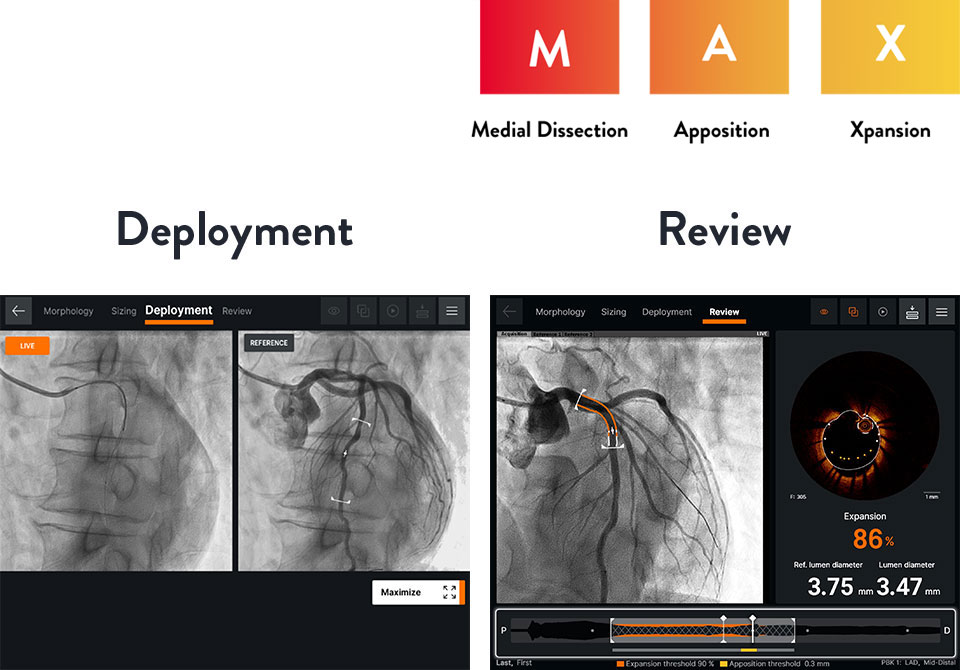
Act Decisively
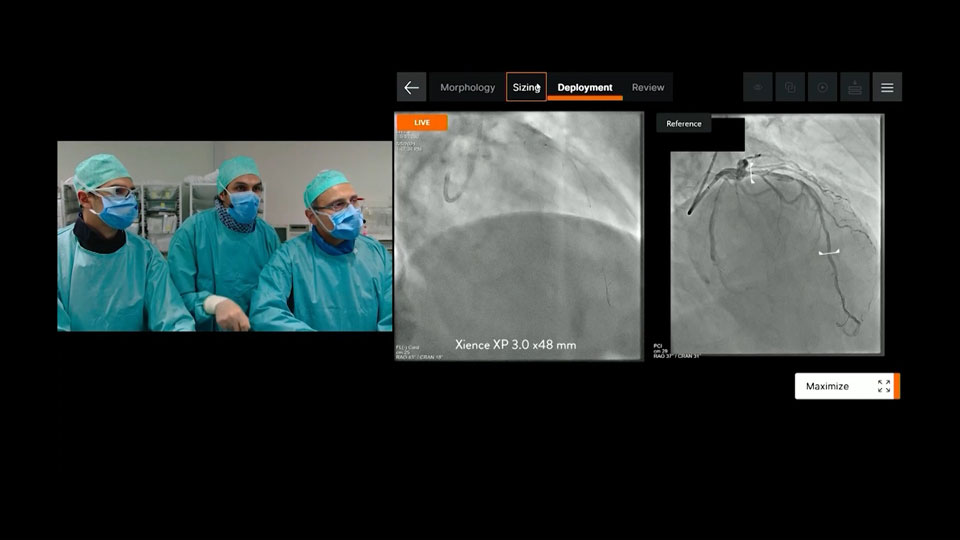
Deployment
Ultreon™ Software provides side-by-side viewing of live and co-registered angio to help physicians guide precise stent deployment.7
- Helps to ensure complete lesion coverage and avoid stent edges in high-risk morphology9
Watch how to use Ultreon™ 1.0 Software for stent deployment
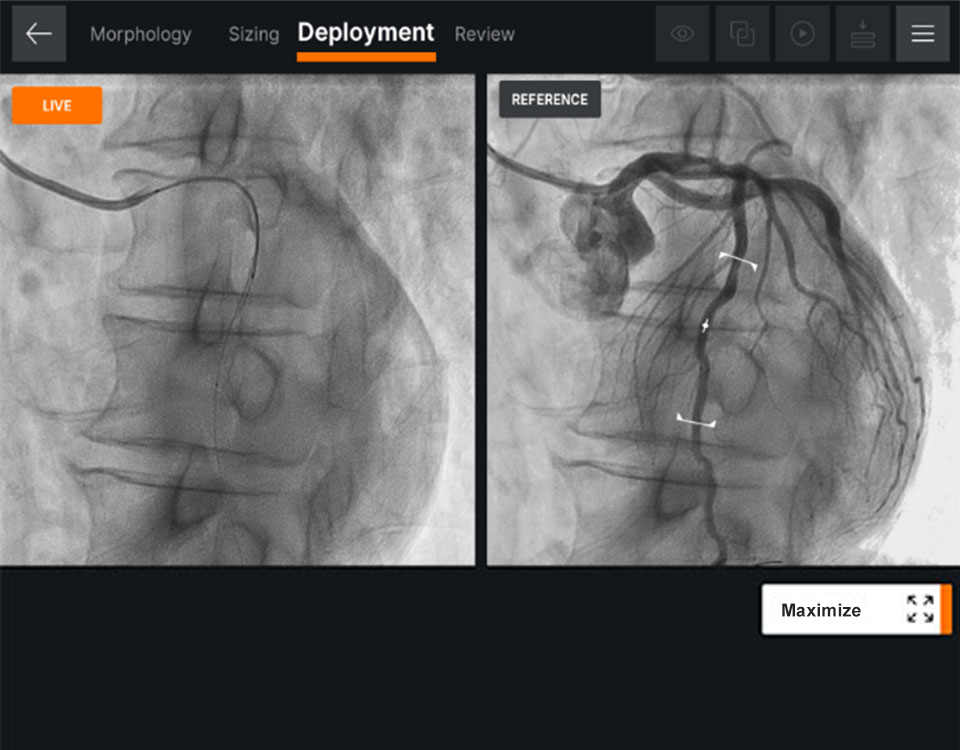
Ultreon™ 1.0 Software interface for stent deployment
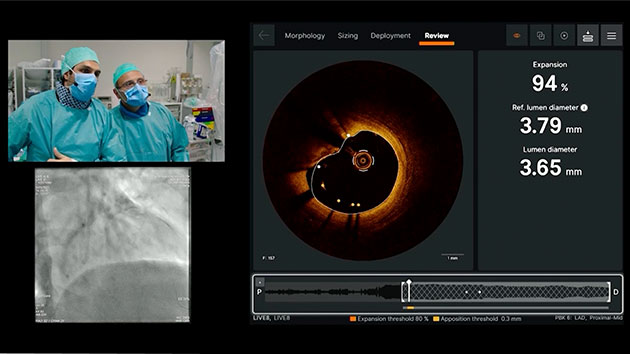
Review
Ultreon™ Software helps to ensure optimal stent expansion and apposition with instantaneous display of expansion and apposition results.7
Watch how to use Ultreon™ 1.0 Software to optimize stent placement
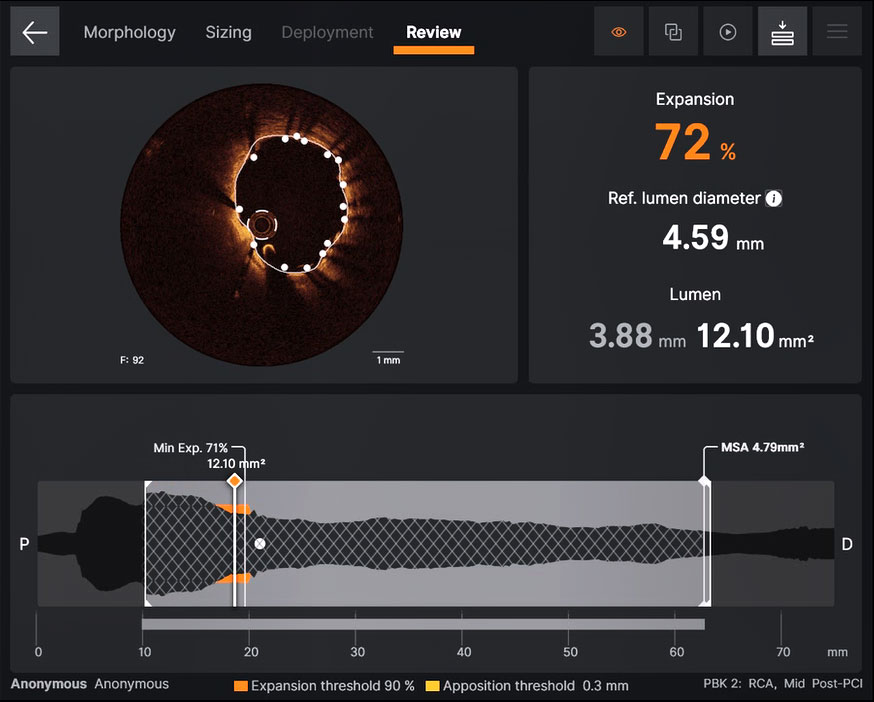
Ultreon™ 1.0 Software interface for review assessment
References
- Data on file at Abbott.
- Zhang J, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: the ULTIMATE trial. J Am Coll Cardiol. 2018;72(24):3126-3137. doi: 10.1016/j.jacc.2018.09.013.
- Hong M, et al. IVUS-XPL 5 Year Outcomes, TCT 2019.
- Jones et al. JACC Cardiovascular Interventions, 2018, vol 11 (14). “Angiography Alone Versus Angiography Plus Optical Coherence Tomography to Guide Percutaneous Coronary Intervention – Outcomes From the Pan-London PCI Cohort”.
- Reyes, M. The next innovation in PCI is not a stent. The value of OCT. CathLab Digest. Oct 6, 2019. Volume 27, Issue 10.
- Bezerra H, et al. Analysis of changes in decision-making process during optical coherence tomography-guided percutaneous coronary interventions: Insights from the LightLab Intiative. EuroPCR 2020.
- Ultreon™ 1.0 Software Instructions for Use (IFU). Refer to IFU for additional information.
- Prati, F. et al. The CLI-OPCI II Study. JACC: Cardiovascular Imaging, 2015: Vol 8, No. 11:1297-305.
- Kubo T, et al. Application of optical coherence tomography in percutaneous coronary intervention. Circ J. 2012;76(9):2076-2083. doi: 10.1253/circj.cj-12-0828.
MAT-2102546 v6.0