Perclose™ ProStyle™ Suture-Mediated Closure for EP Ablation Procedures
The Perclose™ ProStyle™ Suture-Mediated Closure and Repair (SMCR) System enables an improved post-cardiac ablation experience:
- Immediate and durable hemostasis with >96% freedom from major access site-related complications at 30 days1,Δ
- Enhanced patient comfort and satisfaction1,13
- Rapid ambulation and same-day discharge1
PercloseTM ProStyleTM has the broadest indication* for venous and arterial sheaths.
In addition, this vessel closure device also has a proven and trusted track record from more than 20 million repairs.3
Learn how you can finish your procedure with confidence with the Perclose™ ProStyle™ SMCR System.

Rethinking Access Site Management:
Before and After Choosing the Perclose™ ProStyle™ Device
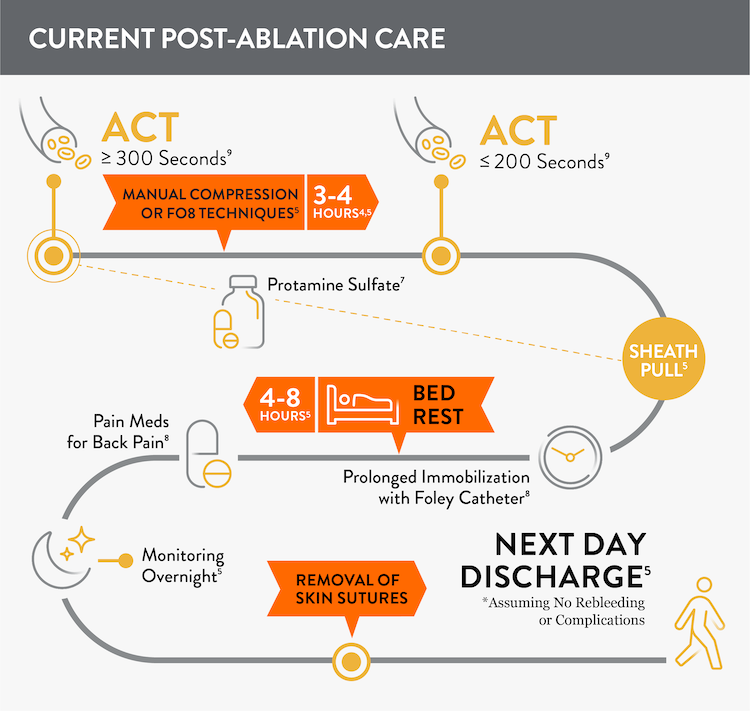
Before: More Closure-Related Interventions Mean More Staff Time
Prior to adopting Perclose™ ProStyle™ SMCR System, atrial fibrillation, including pulsed field ablation or other procedures may find that patients require:
- Multiple venous sheaths and access sites4
- Uninterrupted anticoagulation: activated clotting time (ACT) ≥ 300 seconds5,6
- Manual compression at groin access site for up to 30 minutes5
- Protamine sulfate to reverse the effects of heparin7
- Figure-of-eight (FO8) to maintain hemostasis5
- Prolonged immobilization/bedrest of 4-12 hours to prevent bleeding and complications5
- A Foley catheter8
- Discharge 245-726 hours after the procedure
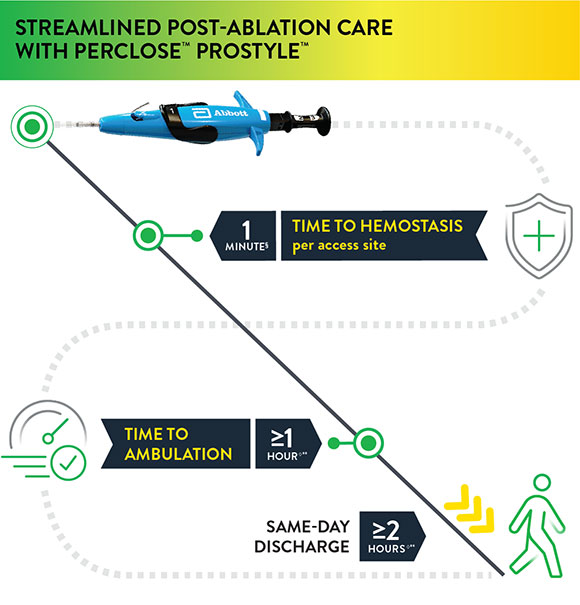
Using Perclose™ ProStyle™ Device: Improved EP Lab Workflow, Enhanced Patient Experience
The Perclose™ ProStyle™ closure device transforms an otherwise lengthy patient recovery to a shorter recovery time, which in turn leads to a positive patient experience:
- Time to hemostasis (TTH) is 1 minute1§ per access site
- Patient may sit up immediately; no lay-flat restrictions1
- Time to ambulation (TTA) is >/1 hour1◊**
- Patient may be eligible for same-day discharge ≥ 2 hours1◊**
§observed median time to hemostasis per access site (DUS) IDE Trial
◊As per the IFU, patients who have undergone cardiac arrhythmia treatments with multiple access sites in a single femoral vein of one or both limbs may be ambulated one hour or more and may be eligible for same-day discharge two hours or more after successful closures with Perclose™ devices based on the judgment of the physician.
The rapid time to hemostasis allows EPs to verify the status of the closure while the patient is still under their care1, enhancing confidence in the entire procedure from access to closure. Moreover, it’s potentially beneficial to the EP lab and hospital staff when patients are quickly ambulating, freeing up beds, and discharged the same day.

Impact of Faster Hemostasis and Patient Discharge on the EP Lab: Potentially Greater Efficiency, Lower Costs, Better Patient Experience
Safety and effectiveness for closing multiple common femoral venous access sites per limb was demonstrated in a duplex ultrasound (DUS) IDE trial and three real-world investigator sponsored studies (ISS) with over 1,000 combined patients.
Click "Learn More" to find out more about these studies: Perclose Multi-Access Duplex Ultrasound (DUS) IDE Trial, Vascular Closure for Cardiac Ablation Registry (VACCAR), Emory School of Medicine (ESM) Study, Santa Barbara Cottage Hospital (SBCH) Study
The use of Perclose™ ProStyle™ Suture-Mediated Closure and Repair System can help:
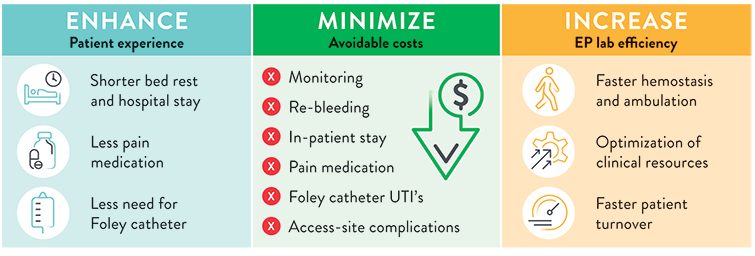
SOURCE: S. Verma. Adopting a Strategy of Early Ambulation and Same-Day Discharge for Atrial Fibrillation Ablation Cases - EP Lab Digest - May 2019.

The Benefits of Suture-Mediated Repair for Vessel Closure
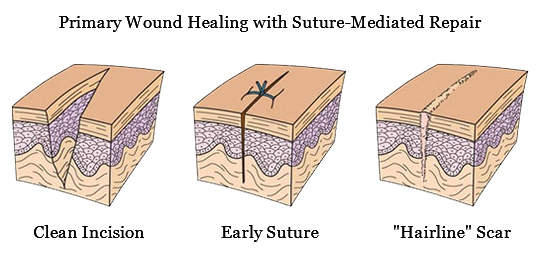
The Perclose™ ProStyle™ closure device achieves rapid hemostasis of femoral access sites by approximating the edges of the vessel wall with a surgical suture. The benefits of suture-mediated repair include promoting primary intention healing with less scarring14 and decreased time to hemostasis, ambulation, and patient discharge.15,16
Suture-Mediated Repair with Perclose™ ProStyle™
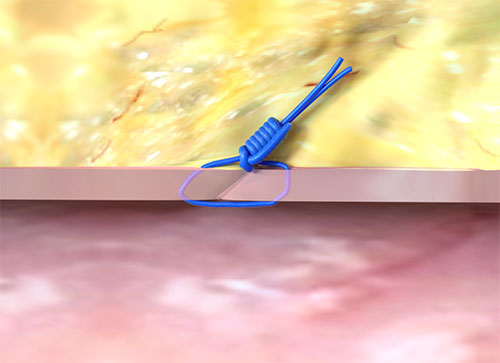
Primary Intention Healing

Frequently Asked Questions
Can Perclose™ ProStyle™ be used as the vessel closure device with Pulsed Field Ablation (PFA) Systems treating atrial fibrillation?
Yes, Perclose™ ProStyle™ can be used in conjunction with a variety of atrial fibrillation ablation techniques, including Pulsed Field Ablation (PFA) Systems
Why would I want to use a vessel closure device (VCD) in a vein?
Using a VCD has several advantages given the following factors during EP procedures such as AF ablations:
- Physicians often have multiple access sites to manage, even some involving large caliber venous sheaths.4
- Patients often receive full-dose peri-procedural anticoagulation, and this can make complete hemostasis a challenging and lengthy process, requiring prolonged immobilization.5,6
- The typical EP lab post-procedure process requires the patient to remain immobilized for prolonged periods of time, which is a source of patient discomfort.5
All of these issues are mitigated when using the Perclose™ ProStyle™ closure device.
Is Same-Day Discharge after AF ablations safe?
Yes, Same-Day Discharge has been shown to be safe, and it is being used to reduce the total cost of care and to enhance the patient experience. The use of vessel closure devices makes it possible for hospitals to implement Same-Day Discharge.13,16,17,18
What makes Perclose™ ProStyle™ SMCR System different from other vascular closure devices?
With the Perclose™ ProStyle™ device you can achieve and confirm complete hemostasis on the table with a suture-mediated repair of the access site. Other advantages of the Perclose™ ProStyle™ SMCR System include:
- The broadest indication* for use in both common femoral veins and arteries
- No ACT-level requirements, so reversal of heparin is not required in order to achieve immediate and durable hemostasis1,8
- Patient may sit-up immediately after successful closure with Perclose™ device1
Find out more about primary healing with the Perclose ProStyle™ vessel closure device.
For what range of sheath sizes and devices can the Perclose™ ProStyle™ SMCR System be used?
Does the Perclose™ ProStyle™ device come in different sizes?
No, there is only one Perclose™ ProStyle™ SMCR System. Multiple Perclose™ ProStyle™ devices can be used, if necessary, for large-bore vascular closure.
How quickly can a patient be mobilized, ambulated, and discharged when using the Perclose™ ProStyle™ closure device?
Because this device achieves immediate and durable hemostasis, patients may sit-up immediately in bed. For cardiac arrhythmia treatments with multiple access sites, patients may be ambulated in as little as 1 hour and be eligible for same-day discharge as early as 2 hours after closure using Perclose™ devices.1
How does the Perclose™ ProStyle™ SMCR System achieve immediate and durable hemostasis?
It achieves hemostasis by approximating the edges of the vessel wall with a surgical suture, allowing primary intention healing to begin. Primary intention healing minimizes scarring and allows for immediate reaccess if needed. View primary intention healing images with vessel closure device.1,14
What kind of training is available to begin using the Perclose™ ProStyle™ SMCR System?
Contact your local Abbott representative for a training opportunity.
What is the "Pre-Close" Technique?
The "Pre-Close" Technique involves the Perclose™ ProStyle™ suture being placed around the access site before the index procedure, and it is required before using sheath sizes > 14F.1
See Deployment and Instructions for Use for additional information.
Visit the official Perclose™ ProStyle™ website for more information on the features, deployment, clinical data, Perclose™ ProStyle™ videos, and ordering information related to Perclose™ ProStyle™ SMCR System.
References
¥ At the time of publication.
† Indicated for closing multiple access sites in the same common femoral vein.
*As compared to Angio-Seal‡, ExoSeal‡, Celt ACD‡, MANTA‡, Mynx‡, Vascade‡. Data on file at Abbott.
** after successful close with Perclose device(s) in patients who have undergone cardiac arrhythmia treatments with multiple common femoral venous access sites
***observed in a duplex ultrasound (DUS) IDE trial and two real-world investigator sponsored studies.
- Perclose™ ProStyle™ Suture-Mediated Closure and Repair (SMCR) System Instructions for Use (IFU). Refer to IFU for additional information.
- Sekhar A, et al. Femoral arterial closure using ProGlide™ is more efficacious and cost-effective when ambulating early following cardiac catheterization. Int J Cardiol Heart Vasc. 2016;13:6-13. doi: 10.1016/j.ijcha.2016.09.002.
- 15 million+ repairs based on August 2022 Finance Report. Data on file at Abbott.
- Gupta S. I Just Need Some Closure: Getting Past Using Manual Compression After Ablation. HRS 2018.
- Lakshmanadoss U, et al. Figure-of-eight suture for venous hemostasis in fully anticoagulated patients after atrial fibrillation catheter ablation. Indian Pacing Electrophysiol J. 2017;17:134-139. doi: 10.1016/j.ipej.2017.02.003
- Okada M, et al. Efficacy and safety of figure-of-eight suture for hemostasis after RFCA for AF. Circ J. 2018;82:956-964. doi: 10.1253/circj.CJ-17-1213.
- Ghannam M, et al. Protamine to expedite vascular hemostasis after catheter ablation of atrial fibrillation: A randomized controlled trial. Heart Rhythm. 2018;15(11):1642-1647. doi: 10.1016/j.hrthm.2018.06.045.
- Mohanty S, et al. Venous access-site closure with vascular closure device vs. manual compression in patients undergoing catheter ablation or left atrial appendage occlusion under uninterrupted anticoagulation. EP Europace. 2019;21:1048-1054. doi.org/10.1093/europace/euz004.
- Calkins H, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444. http://dx.doi.org/10.1016/j.hrthm.2017.05.012.
- Kar, S., et al, The Use of Perclose ProGlide Suture-Mediated Closure (SMC) Device for Venous Access-Site Closure Up to 24F Sheaths. CRT 2018.
- Mahadaven VS, et al. Pre-closure of femoral venous access sites used for large-sized sheath insertion with the Perclose device in adults undergoing cardiac intervention. Heart. 2008;94:571-572. doi.org/10.1136/hrt.2006.095935.
- Sairaku A, et al. Rapid hemostasis at the femoral venous access site using a novel hemostatic pad containing kaolin after atrial fibrillation ablation. J Interv Card Electrophysiol. 2011;31:157-164.
- Verma S. Adopting a strategy of early ambulation and same-day discharge for atrial fibrillation ablation cases. EP Lab Digest. 2019;19(5).
- Mercandetti M. Wound Healing and Repair. Medscape. Accessed February 26, 2020. https://emedicine.medscape.com/article/1298129-overview
- Bhatt DL, et al. Successful “pre-closure" of 7Fr and 8Fr femoral arteriotomies with a 6Fr suture-based device (the Multicenter Interventional Closer Registry). Am J Cardiol. 2002;89:777-779.
- Fabbricatore D et al. Ambulatory PV isolation workflow using suture-mediated vascular closure devices: a prospective observational cohort study. (PRO-PVI Study).
- Bartoletti S, Mann M, Gupta A, et al. Same‐day discharge in selected patients undergoing atrial fibrillation ablation. Pacing Clin Electrophysiol. 2019;42:1448-1455.
- Deyell M, Macle L, Khairy P, et al. The efficacy of a same-day discharge protocol after atrial fibrillation ablation. Canadian J Cardiol. 2018;34:(10 suppl):S84. doi:https://doi.org/10.1016/j.cjca.2018.07.281.
- Data on file at Abbott.
- Verma, S., et al, Feasibility and Safety of Same Day Discharge for Patients Undergoing Atrial Fibrillation (AF) Ablation in a Community Hospital Setting. HRS 2020 Science Online, May 2020.
MAT-2004330 v7.0
Important Safety Information
Perclose™ ProStyle™ Suture-Mediated Closure and Repair (SMCR) System

Indications:
The Perclose™ ProStyle™ Suture-Mediated Closure and Repair System is indicated for the percutaneous delivery of suture for closing the common femoral artery and vein access sites of patients who have undergone diagnostic or interventional catheterization procedures.
The Perclose™ ProStyle™ SMCR System is indicated for closing the common femoral vein in single or multiple access sites per limb.
The Perclose™ ProStyle™ SMCR System is used without or, if required, with adjunctive manual compression.
For access sites in the common femoral artery using 5F to 21F sheaths. For arterial sheath sizes greater than 8F, at least two devices and the pre-close technique are required.
For access sites in the common femoral vein using 5F to 24F sheaths. For venous sheath sizes greater than 14F, at least two devices and the pre-close technique are required.
Caution:
Federal law restricts this medical device to sale by or on the order of a physician (or allied healthcare professionals, authorized by, or under the direction of, such physicians) who is trained in diagnostic and / or interventional catheterization procedures and who has been trained by an authorized representative of Abbott.
Prior to use, the operator must review the Instructions for Use and be familiar with the deployment techniques associated with the use of this device.
During closure of access sites using a procedural sheath greater than 8F, it is recommended that a vascular surgeon or a surgeon with vascular training be available in case surgical conversion to control bleeding and to repair the vessel is needed.
Contraindications:
There are no known contraindications to the use of this device.
Warnings:
Do not use the Perclose™ ProStyle™ SMCR System if the packaging or sterile barrier has been previously opened or damaged or if the components appear to be damaged or defective.
DO NOT RESTERILIZE OR REUSE. The Perclose™ ProStyle™ SMCR System is intended for single use only.
Do not use the Perclose™ ProStyle™ SMCR System if the sterile field has been broken where bacterial contamination of the sheath or surrounding tissues may have occurred, since such a broken sterile field may result in infection.
Do not use the Perclose™ ProStyle™ SMCR System if the puncture site is located above the most inferior border of the inferior epigastric artery (IEA) and / or above the inguinal ligament based upon bony landmarks, since such a puncture site may result in a retroperitoneal hematoma. Perform a femoral angiogram to verify the location of the puncture site. Note: This may require both a right anterior oblique (RAO) and left anterior oblique (LAO) angiogram to adequately visualize where the sheath enters the femoral vessel.
Do not use the Perclose™ ProStyle™ SMCR System in arterial or venous access if the puncture is through the posterior wall or if there are multiple punctures in the same access site, since such punctures may result in a hematoma or retroperitoneal bleed.
Do not use the Perclose™ ProStyle™ SMCR System if the puncture site is located in the superficial femoral artery or the profunda femoris artery, or the bifurcation of these vessels, since such puncture sites may result in a pseudoaneurysm, intimal dissection, or an acute vessel closure (thrombosis of small artery lumen). Perform a femoral angiogram to verify the location of the puncture site. Note: This may require both a right anterior oblique (RAO) and left anterior oblique (LAO) angiogram to adequately visualize where the sheath enters the femoral vessel.
Precautions:
- Prior to use, inspect the Perclose™ ProStyle™ SMCR System to ensure that the sterile packaging has not been damaged during shipment. Examine all components prior to use to verify proper function. Exercise care during device handling to reduce the possibility of accidental device breakage.
- As with all catheter-based procedures, infection is a possibility. Observe sterile technique at all times when using the Perclose™ ProStyle™ SMCR System. Employ appropriate groin management, as per hospital protocol, post-procedure, and post-hospital discharge to prevent infection.
- Use a single wall puncture technique. Do not puncture the posterior wall of the vessel in arterial and venous access.
- Do not deploy the Perclose™ ProStyle™ Device at an elevated angle against resistance as this may cause a cuff miss or device breakage.
- There are no reaccess restrictions if previous arteriotomy / venotomy repairs were achieved with Abbott Medical SMC or SMCR systems.
- If significant blood flow is present around the Perclose™ ProStyle™ Device, do not deploy needles. Remove the device over a 0.038" (0.97 mm) (or smaller) guide wire and insert an appropriately sized sheath.
- Prior to depressing the plunger to advance the needles, stabilize the device by the body to ensure the foot is apposed to the vessel wall and the device does not twist during deployment. Twisting (torquing) of the device could lead to needle deflection resulting in a cuff miss. Do not use excessive force or repeatedly depress the plunger. Excessive force on the plunger during deployment could potentially cause breakage of the device, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- Do not apply excessive force to the lever when opening the foot and returning the foot to its original position down to the body of the device. Do not attempt to remove the device without closing the lever. Excessive force on the lever or attempting to remove the device without closing the lever could cause breakage of the device and / or lead to vessel trauma, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- Do not advance or withdraw the Perclose™ ProStyle™ Device against resistance until the cause of that resistance has been determined. Excessive force used to advance or torque the Perclose™ ProStyle™ Device should be avoided, as this may lead to significant vessel damage and / or breakage of the device, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- If excessive resistance in advancing the Perclose™ ProStyle™ Device is encountered, withdraw the device over a 0.038" (0.97 mm) (or smaller) guide wire and reinsert the introducer sheath or use manual compression.
- Remove the Perclose™ ProStyle™ sheath before tightening the suture. Failure to remove the sheath prior to tightening the suture may result in detachment of the tip of the sheath.
- Care should be taken to avoid damage to the suture from handling. Avoid crushing damage due to application of surgical instruments such as clamps, forceps or needle holders.
- For catheterization procedures using a 5F – 8F procedural sheath, use manual compression in the event that bleeding from the femoral access site persists after the use of the Perclose™ ProStyle™ SMCR System to obtain hemostasis.
- For catheterization procedures using a procedural sheath > 8F, use manual compression, compression assisted devices, surgical repair, and / or other appropriate treatment methods in the event that bleeding from the femoral access site persists after the use of the Perclose™ ProStyle™ SMCR System to obtain hemostasis.
- For catheterization procedures using a procedural sheath > 8F, where the operating physician is not a vascular surgeon, it is recommended that a vascular surgeon or a surgeon with vascular training be available during the procedure to perform any necessary vascular surgical intervention.
- If the Perclose™ ProStyle™ Device is used to close and repair multiple access sites in the same vessel, space the access sites apart adequately to minimize sheath-device interference.
Potential Adverse Events:
Potential adverse events associated with use of vessel closure devices may include, but are not limited to, the following:
- Allergic reaction or hypersensitivity to device components
- Vascular access complications which may require transfusion or vessel repair, including:
- Anemia
- Aneurysm
- Arteriovenous fistula
- Bleeding / hemorrhage / re-bleeding
- Bruising
- Hematoma
- Embolism
- Inflammation
- Intimal tear / dissection
- Perforation
- Pseudoaneurysm
- Retroperitoneal hematoma / bleeding
- Scar formation
- Wound dehiscence
- Cardiac arrhythmias (including conduction disorders, atrial and ventricular arrhythmias)
- Atrial arrhythmias
- Ventricular arrhythmias
- Femoral artery / venous complications which may require additional intervention, including:
- Arterial / venous stenosis
- Arterial / venous occlusion
- Arteriovenous fistula
- Intimal tear / dissection
- Ischemia distal to closure site
- Nerve injury
- Numbness
- Thrombus formation
- Vascular injury
- Venous thromboembolism (including deep vein thrombosis, pulmonary embolism, post-procedure pulmonary embolism)
- Infection - local or systemic
- Pain
- Hemodynamic instability:
- Hypotension / hypertension
- Vasovagal episode
- Death
- Device complications
- Device failure
- Device malfunction
MAT-2100368 v4.0
