
Percutaneous Closure of Large Access Sites
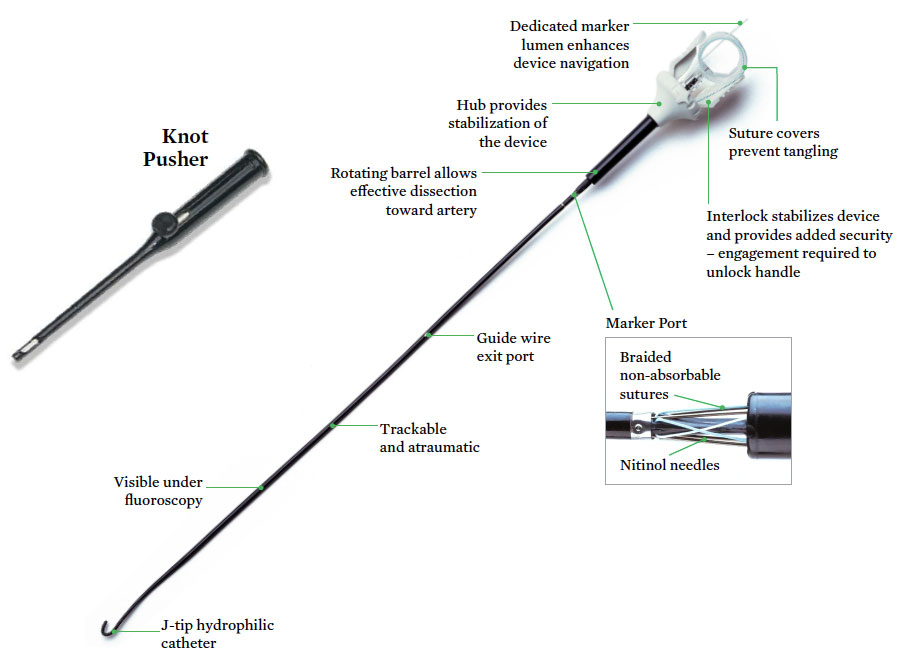
ProStar™ XL Percutaneous Vascular Surgical System offers percutaneous surgical repair of femoral artery access sites 8.5-10F. Other features of the Prostar™ XL PVS System
- Trackable sheath with atraumatic tip
- Rotational Hub allows for easy dilation during advancement
- Providing secure vascular closure
Close Access Sites up to 10F*
- 8.5-10F
- Diagnostic and interventional procedures
- No known contraindications
- No re-access restrictions
Secure Closure
- Uses two braided polyester sutures (Tevdek) and four nitinol needles results in a secure close
- Flexibility to close over-the-wire to maintain vessel access
Data on file at Abbott.
*Prostar XL Percutaneous Vascular Surgical System – Instructions for Use (IFU). Refer to IFU for additional information.
MAT-2207294 v2.0
Important Safety Information
Prostar™ XL
Percutaneous Vascular Surgical (PVS) System

INDICATIONS FOR USE
The Prostar™ XL PVS System is indicated for the percutaneous delivery of sutures for closing the common femoral artery access site and reducing the time to hemostasis and time to ambulation (patient walks ten feet) of patients who have undergone catheterization procedures using 8.5F to 10F sheaths. (Refer to PRECAUTIONS, SPECIAL PATIENT POPULATIONS sections).
CONTRAINDICATIONS
None known.
WARNINGS
The outer pouch of the Prostar™ XL PVS System and the individual accessories provides the sterile barrier. Do not use the Prostar™ PVS System or accessories if the packaging or sterile barrier have been previously opened or damaged, or if the components appear to be damaged or defective.
DO NOT RESTERILIZE OR REUSE. The Prostar™ XL PVS System and accessories are intended for single use only.
Do not use the Prostar™ XL PVS System if the puncture site is proximal to the inguinal ligament as this may result in a retroperitoneal hematoma.
PRECAUTIONS
- The Prostar™ XL PVS device and accessories should only be used by physicians (or other healthcare professionals authorized by or under the direction of such physicians) after they have been trained in the use of the Prostar™ XL PVS System and accessories, e.g., participation in a Prostar™ XL PVS System training program or equivalent.
- Observe sterile technique at all times when using the Prostar™ XL PVS System. Employ appropriate groin management, as per hospital protocol, post procedure and post hospital discharge to prevent infection.
- Use a single wall puncture technique. Do not puncture the posterior wall of the artery.
- Adequate knot security requires accepted surgical technique as warranted by surgical circumstances and the experience of the operator.
- There are no reaccess restrictions if previous arteriotomy repairs were achieved with an Abbott Vascular Suture Mediated Device.
- Do not insert the Prostar™ XL device into the femoral artery at an angle greater than 45 degrees to the longitudinal plane of the artery.
- Do not advance or withdraw the Prostar™ XL device against resistance until the cause of that resistance has been determined (see CLINICAL PROCEDURE-Device Placement section). Excessive force used to advance or torque the Prostar™ XL device should be avoided as it may lead to significant arterial damage and / or breakage of the device, which may necessitate intervention and / or surgical removal of the device and arterial repair.
- If excessive resistance in advancing the Prostar™ XL device is encountered, withdraw the Prostar™ XL device over a 0.038” (or smaller) guide wire and reinsert the introducer sheath or use conventional compression therapy.
- In the event suture breakage occurs after an initial knot has been tied, care should be taken to avoid excessive force if the reintroduction of the Prostar™ XL device or introducer sheath is required. Any resistance to introduction should result in advancement of an introducer sheath small enough to be introduced without undue force.
- If significant blood flow is evident through or around the barrel of the Prostar™ XL device, do not deploy needles. Remove the Prostar™ XL device over a 0.038” (or smaller) guide wire and insert an appropriately sized introducer sheath.
- Remove the Prostar™ XL sheath before tightening the suture. Failure to remove the sheath prior to tightening the suture may result in detachment of the tip of the sheath.
- Do not attempt to re-deploy Prostar™ XL needles after the needles have been “backed-down” into the sheath (refer to the TECHNIQUE FOR NEEDLE BACK-DOWN section).
- In the event bleeding from the femoral access site persists after the use of the Prostar™ XL device and accessories, use conventional compression therapy.
ADVERSE EVENTS
The following adverse events have been reported and may occur include:
- Device Malfunction
- Device Complication
- Vascular Repair
- Ultrasound Guided Compression
- Transfusion
- Infection Requiring IV Antibiotics
- Hematoma > 6 cm
- AV Fistula
- Nerve Injury
- Pseudoaneurysm
- Deep Vein Thrombosis
- Late Bleeding
- Wound Dehiscence
- Vessel Laceration
- Local Pulse Deficits or Ischemia
- Embolization
- Transitory Local Irritation
- Nerve Injury
- Vascular Spasm
MAT-2114591 v3.0
