Amplatzer™ Vascular Plug II
(AVP II)
Deployment
Note: This section should not be used in place of the Instructions for Use. This section only provides an overview of the procedure. Refer to the Instructions for Use for additional information.1
Access the vessel and perform an angiogram using standard technique to measure the diameter of the vessel at the desired occlusion site.
Select a vascular plug with a diameter approximately 30-50% larger than the vessel diameter at the occlusion site. Ensure that the occlusion site has sufficient length so the deployed device length will not obstruct other vessels or anatomical structures.
Deployment Steps

| Deployment Steps | |
|---|---|
| 1 | Flush hoop dispenser and loader with sterile saline until fluid exits the distal tip to purge air from the loader. |
| 2 | Remove device (in loader) and delivery wire from the hoop dispenser. |
| 3 | Select a delivery catheter with an internal diameter that will accommodate the selected device. The original access catheter may be used if the internal diameter is sufficient for the selected device. Prepare the catheter for use (according to the manufacturer’s instructions for use). |
| 4 | Advance the delivery catheter over the guidewire until the distal tip of the catheter is at the distal edge of the occlusion site (according to the manufacturer’s instructions for use). |
| 5 | Remove the guidewire. |
| 6 | Insert loader into the delivery catheter through a Y-connector or hemostasis valve. |
| 7 | Allow blood backflow or aspirate the system to ensure air is purged from the catheter and loader. |
| 8 | Do not over tighten Y-connector to avoid damaging loader. |
| 9 | Advance the delivery wire to advance the device to the distal end of the delivery catheter. Do not twist or rotate the delivery wire during advancement to ensure device does not prematurely detach. |
| 10 | Hold delivery wire in place and slowly retract the delivery catheter to deploy the device at the occlusion site. |
| 11 | Verify position of the device using the radiopaque marker bands. ~ If unsatisfactory, stabilize the wire and re-advance the delivery catheter until the device is completely within the catheter. Reposition and deploy, or remove the device from the patient. ~ If satisfactory, attach the plastic visea to the wire and rotate the delivery wire counterclockwise until it separates from the device." |
| 12 | Remove delivery catheter and wire from the patient. |
Steps 1-3


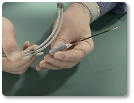
Steps 4-5
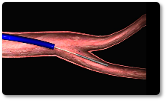
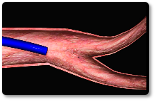
Steps 6-9
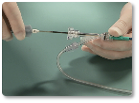
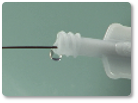
Steps 10-12
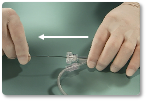
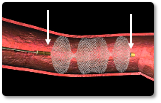

References
- Amplatzer™ Vascular Plug and Vascular Plug II - Instructions for Use
MAT-2002814 v1.0
AMPLATZER™ Vascular Plug II

Intended Use: The AMPLATZER™ Vascular Plug II is indicated for arterial and venous embolizations in the peripheral vasculature.
Contraindications: None known.
Warnings: The safety and effectiveness of this device for cardiac uses (for example, patent ductus arteriosus or paravalvular leak closures) and neurological uses have not been established.
Precautions:
- This device was sterilized with ethylene oxide and is for single use only. Do not reuse or resterilize this device. Attempts to resterilize this device can cause a malfunction, insufficient sterilization, or harm to the patient.
- Use on or before the last day of the expiration month that is printed on the product packaging label.
- Patients with nickel allergy can experience an allergic reaction to this device.
- Do not use this device if the sterile package is open or damaged.
- This device should be used only by physicians who are trained in standard endovascular techniques. The physician should determine which patients are candidates for procedures that use this device.
- The physician should exercise clinical judgment in situations that involve the use of anticoagulants or antiplatelet drugs before, during, and/or after the use of this device.
- Store in a dry place.
MRI Safety Information:
| Device Name | Amplatzer™ Vascular Plug II |
| Static Magnetic Field Strength (B0) | 1.5 T or 3.0 T |
| Maximum Field Spatial Gradient | 19T/m (1900 gauss/cm) |
| RF Excitation | Circularly Polarized (CP) |
| RF Transmit Coil Type | Body Coil |
| Operating Mode | Normal Operating Mode |
| Maximum Whole Body SAR | 2.0W/kg (Normal Operating Mode) |
| Maximum Head SAR | N/A |
| Scan Duration | 2.0W/kg whole-body-averaged SAR for 15 minutes of continuous scanning |
| MR Image Artifact | The presence of this implant may produce an image artifact. |
Potential Adverse Events: Potential complications include, but are not limited to: death, migration of the device, stroke, or vessel perforation.
State of California (USA) Only:
WARNING: This product can expose you to chemicals including ethylene oxide, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information, go to www.P65Warnings.ca.gov.
MAT-2004317 v4.0


