Accessories
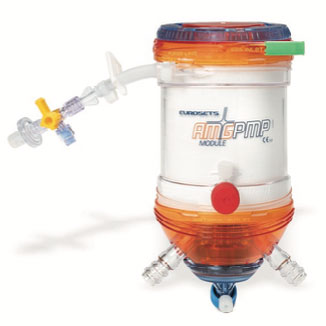
AMG PMP Oxygenators
Designed for hemocompabitiltity, Abbott’s portfolio of AMG PMP Oxygenators utilizes microporous polymethylpentene (PMP) hollow fibers coated with a propriety phosphorylcholine (PC) coating to promote low thrombogenicity and inflammatory responses. They also feature integrated, stainless steel heat exchangers for blood temperature management, low priming volumes and reliable gas exchange performance.
Pre-connected Pack
Experience optimal convenience with a pre-connected circuit with the CentriMag™ Pre-connected Pack. The pack consists of the CentriMag™ Blood Pump, the CentriMag™ Adult Oxygenator, a priming bag, phosphorylcholine (PC) coated tubing and accessories for a complete circuit that’s ready to go for cardiopulmonary bypass.
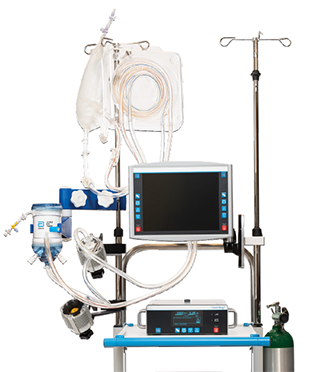
CentriMag System Transporter
Get patients where they need to be with the next generation CentriMag™ System Transporter. This compact form provides the durability and protection needed to safely transport patients inside or outside the hospital and the versatility to adapt to your transport needs.
- Ability to hold core components including console, pump/motor and oxygenator for convenient transport
- Collapsible rail hooks and detachable sides for placement flexibility
- Compact design for convenient fit into air or ground ambulance
- No ground plate required; CentriMag™ Pump impeller maintains its orientation automatically through Full MagLev™ Flow Technology
- Machined aluminum oxygenator bracket, compatible with all Eurosets AMG PMP Oxygenators and available only from Abbott
- Built on 10 years of transport data with the CentriMag™ Acute Circulatory Support System1
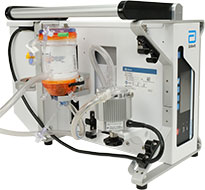
AMG PMP Oxygenator and Oxygenator Holder manufactured by Eurosets s.r.l., and distributed by Abbott.
*PMA approval for 30-day use of CentriMag™ System components include: CentriMag™ Pump, CentriMag™ Console, CentriMag™ Motor, Mag Monitor, flow probe, and CentriMag™ Drainage Cannula and CentriMag™ Return Cannula. Optional accessories include: CentriMag™ System Cart, CentriMag™ System Transporter and Pressure Transducer. PMA approval for 30-day use of CentriMag™ System excludes: PediMag™ Blood Pump and any other pediatric components or accessories.
References
- Corno A, Faulkner G, Harvey C. Mobile extracorporeal membrane oxygenation. ASAIO Journal. 2020. doi:10.1097/MAT.00000000000012786
MAT-2207134 v3.0

Stay Connected