About the CentriMag Acute Circulatory Support System
Excellent hemocompatibility and unparalleled versatility for optimized patient care.
Primed for
ECMO
CentriMag™ Acute Circulatory Support System with Full MagLev™ Flow Technology
The CentriMag System is the only acute circulatory support system approved for 30-day LVAD, RVAD and BiVAD support and ECMO‡. It offers freedom of choice and fits seamlessly into custom circuit designs, and can be used with a variety of cannulation options.

Excellent Survival Outcomes at Discharge
CentriMag™ Blood Pump:
as part of a VA-ECMO Circuit7
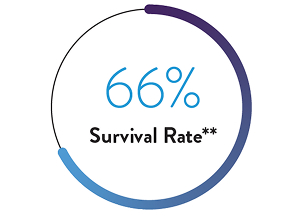
ELSO International Summary:
Survival to Discharge6
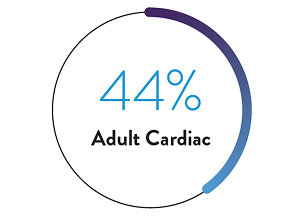
Survival to discharge for other adult support indications includes 54% overall, 30% ECPR and 59% pulmonary.
Full MagLev Flow Technology Promotes Gentle Blood Handling
- Wide blood-flow pathways and absence of seals, bearings and valves minimize blood turbulence and stasis.
- Free-floating magnetically levitated rotor prevents surface-to-surface contact that could cause blood trauma.
- Automatic rotor positioning control adjusts 5,000 times per second to maintain stable and consistent blood flow.
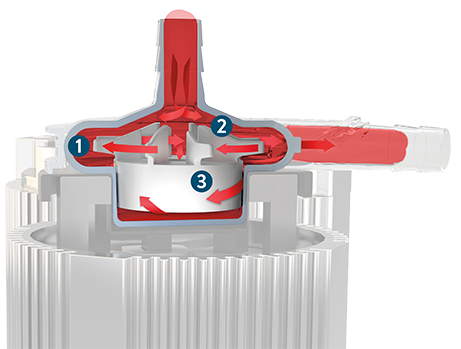
Watch this video to see the CentriMag Blood Pump and Full MagLev Flow Technology in action.
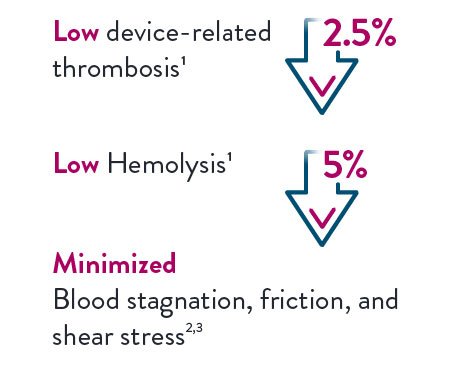
Designed for Optimal Hemocompatibility1,2
Driven by Full MagLev™ Flow Technology, the CentriMag and PediMag Pumps include a free-floating magnetically levitated rotor for a contact-free environment. This innovative technology results in excellent hemodynamics, which can lead to minimized blood stagnation, friction and shear stress.3,4
Addressing the broadest spectrum of clinical challenges
The CentriMag blood pump functions as:
- Part of an ECMO†† circuit for periods > 6 hours
- Temporary circulatory support for periods up to 30 days* to support the left, right, or both ventricles of the heart in patients who fail to wean from cardiopulmonary bypass
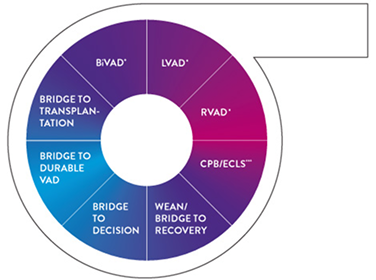
Optimal Patient Care for a Variety of Applications
Trusted for a wide range of patient types and sizes
CentriMag System hardware (console, motor and monitor) is shared between the adult and pediatric pump options.
Adult and pediatric pump options are available.*
Produces flows of up to 10 LMP with fewer rotations per minute.3
*PMA approval for 30-day use of CentriMag System components include: CentriMag™ Pump, CentriMag™ Console, CentriMag™ Motor, Mag Monitor, Flow Probe, and CentriMag™ Drainage Cannula and CentriMag™ Return Cannula. Optional accessories include: CentriMag™ System Cart, CentriMag™ System Transporter and Pressure Transducer. PMA approval for 30-day use of CentriMag™ System excludes: PediMag™ Blood Pump and any other pediatric components or accessories.
† Approved for 30-day use
†† ECMO clearance for >6-hour use is indicated for the CentriMag Blood Pump to be used with: CentriMag Console, CentriMag Motor, Mag Monitor, and Flow Probe. ECMO clearance for the CentriMag Blood Pump is for adult use only and excludes: CentriMag Drainage Cannula and CentriMag Return Cannula, PediMag Blood Pump and any other pediatric components or accessories.
References
- John, R., Liao, K., Lietz, K., Kamdar, F., Colvin-Adams, M., Boyle, A., … Joyce, Lyle. (2007). Experience with the Levitronix CentriMag circulatory support system as a bridge to decision in patients with refractory acute cardiogenic shock and multisystem organ failure. The Journal of Thoracic and Cardiovascular Surgery, 134(2), 351-358. https://dx.doi.org/10.1016/j.jtcvs.2007.01.085
- John, R., Long, J. W., Massey, H. T., Griffith, B. P., Sun, B. C., Tector, A. J., … Joyce, L. D. (2011). Outcomes of a multicenter trial of the Levitronix CentriMag ventricular assist system for short-term circulatory support. The Journal of Thoracic and Cardiovascular Surgery, 141(4), 932-939. https://dx.doi.org/10.1016/j.jtcvs.2010.03.046
- Aziz, T. A., Singh, G., Popjes, E., Stephenson, E., Mulvey, S., Pae, W., & El-Banayosy, A. (2010). Initial experience with CentriMag extracorporeal membrane oxygenation for support of critically ill patients with refractory cardiogenic shock. The Journal of Heart and Lung Transplantation, 29(1), 66-71. https://dx.doi.org/10.1016/j.healun.2009.08.025
- Bhama, J. K., Kornos, R. L., Toyoda, Y., Teuteberg, J. J., McCurry, K. R., & Siegenthaler, M. P. (2009). Clinical experience using the Levitronix CentriMag system for temporary right ventricular mechanical circulatory support. The Journal of Heart and Lung Transformation, 28(9), 971-976. https://www.jhltonline.org/article/S1053-2498(09)00238-1/fulltext
- Takeda K, Garan AR, Ando M, et al. Minimally invasive CentriMag ventricular assist device support integrated with extracorporeal membrane oxygenation in cardiogenic shock patients: a comparison with conventional CentriMag biventricular support configuration. Eur J Cardio-Thorac Surg. 2017;52:1055-1061.
- Worku B, Pak S-W, van Patten D, et al. The CentriMag ventricular assist device in acute heart failure refractory to medical management. J Heart Lung Transplant. 2012;31:611-617.
- Takayama H, Soni L, Kalesan B, et al. Bridge-to-decision therapy with a continuous-flow external ventricular assist device in refractory cardiogenic shock of various causes. Circ Heart Fail. 2014;7:799-806.
MAT-2207132 v5.0
