MAT-2514762 v1.0 | Item approved for U.S. use only. ©2025 Abbott. All Rights Reserved.
Keeping hearts beating more regularly with the most advanced technologies
Harnessing the capabilities of leadless technology, two distinct devices - one for the right atrium and one for the right ventricle – can be implanted without chest incisions or pockets. Explore the streamlined implantation procedures for atrial and ventricular leadless pacing in the single chamber workflows below to gain a deeper understanding of these minimally invasive techniques.
How the AVEIR VR Ventricular Leadless Pacemaker is implanted1
CONNECT patient's skin electrodes to MerlinTM Programmer via the EKG cable and AVEIR Link Module.
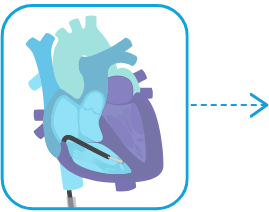
INSERT catheter with attached ventricular pacemaker and navigate to right ventricle.
MAP ventricular implanting location before fixation by touching endocardium with AVEIR VR Leadless Pacemaker's tip electrode.
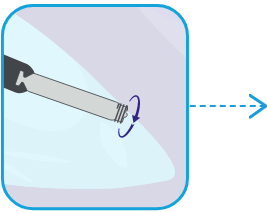
FIXATE the AVEIR VR Leadless Pacemaker to the ventricular endocardium using the screw-in mechanism.
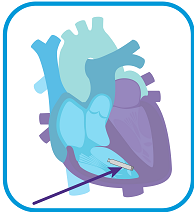
RELEASE the AVEIR VR Leadless Pacemaker and withdraw delivery catheter.
For a more detailed workflow, refer to the AVEIR VR Instructions for Use (IFU) or contact your Abbott Sales Representative for training.
How the AVEIR AR Atrial Leadless Pacemaker is implanted2
.png)
CONNECT patient's skin electrodes to MerlinTM Programmer via the EKG cable and AVEIR Link Module.
.png)
INSERT catheter with attached atrial pacemaker and navigate to right atrium.
.png)
MAP atrial implanting location before fixation by touching endocardium with AVEIR AR Leadless Pacemaker's tip electrode.
.png)
FIXATE the AVEIR AR Leadless Pacemaker to the atrial endocardium using the screw-in mechanism.
.png)
RELEASE the AVEIR AR Leadless Pacemaker and withdraw delivery catheter.
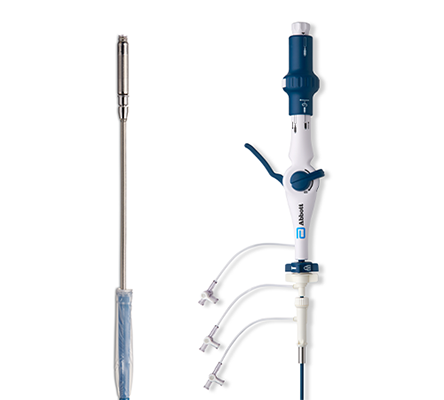
Our Innovative Catheter Technology
AVEIR Delivery and Retrieval Catheters
- Designed for ergonomic, single operator use.
- Steerable catheter with deflection mechanism.1
- Hydrophilic coating on introducer sheath and a choice of 30cm and 50cm lengths.4
- Protective sleeve fully covers the AVEIR Leadless Pacemaker's helix during catheter navigation in order to reduce the risk of damaging the helix or an injury to cardiovascular structures.1,3
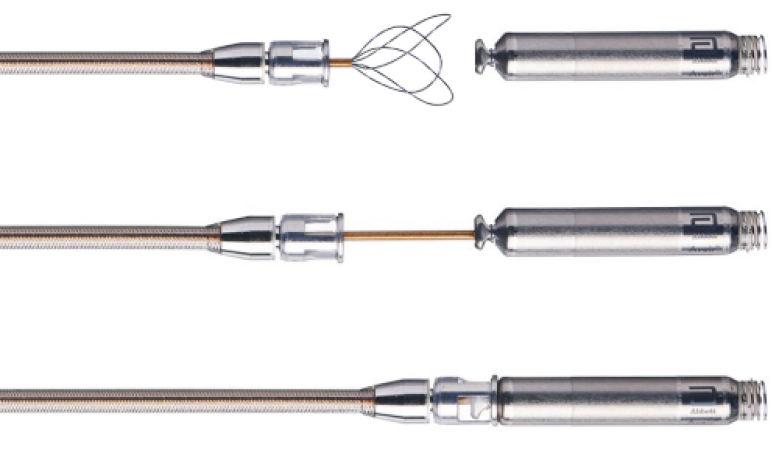
Tri-loop snare re-docking mechanism
Retrieval Catheter Enables Long-Term Retrieval
- AVEIR tri-loop snare re-docking mechanism enables retrieval of the Leadless Pacemaker.
- AVEIR VR and AR Leadless Pacemakers' active fixation helix uses a screw-in mechanism to enable both implantation and long-term retrieval of the Leadless Pacemaker.1,5
- The AVEIR VR Leadless Pacemaker's predicate device has an overall long-term retrieval success rate above 88% with helix fixation through 9 years of retrieval experience.5 Limited data is available for AVEIR VR LP.
ENABLING SAFE MRI SCANS
AVEIR Leadless Pacemakers are MR Conditional for full body scans using a 1.5T or 3T strength MRI scanner.
AV
Stay Informed
Sign up to hear about our technology, education opportunities, and more.
Read the Latest Blog Article
Stay up to date with recent news, product highlights, and case studies.
References
- AVEIR™ VR Leadless Pacemaker and Delivery Catheter IFU. ARTEN600175956
- AVEIR™ Leadless Pacemakers and Delivery Catheter IFU, ARTEN600284235
- AVEIR™ Retrieval Catheter IFU. ARTMT600174816
- AVEIR™ Introducer IFU. ARTEN600174817
- Reddy, VY, et al. Worldwide Experience with Leadless Pacemaker Retrievals at 9 years. Presented at 15th Asia Pacific Heart Rhythm Society (APHRS) Scientific Session; Nov 18-20, 2022; Singapore.
MAT-2115644 v10.0 | Item is approved for US use