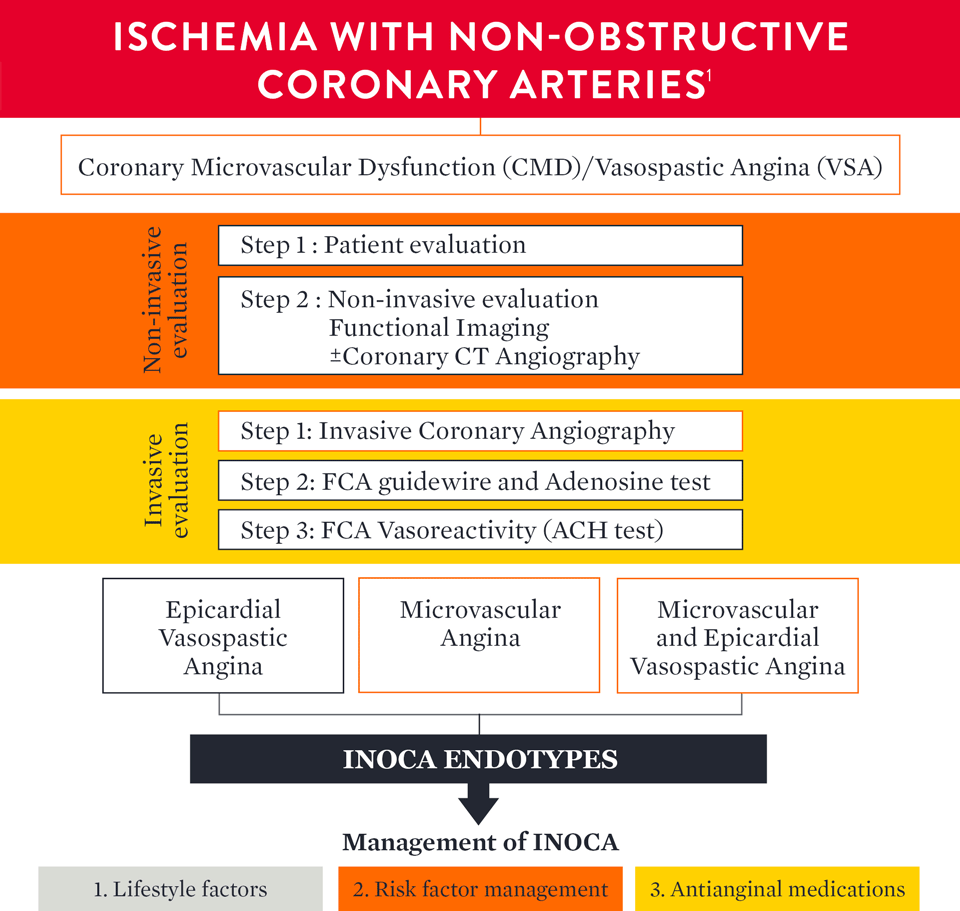
How to Diagnose INOCA
The European Association of Percutaneous Cardiovascular Interventions (EAPCI) Expert Consensus Document defines ischemia and no obstructive coronary artery disease (INOCA) and guidance to its diagnosis and management. The document states that, INOCA patients present with a wide spectrum of symptoms and signs that are often misdiagnosed as non-cardiac, leading to under-diagnosis/investigation and under-treatment.1
Guidelines Recommend Guidewire-Based Measurements
Both American and European guidelines provide a class IIa recommendation for a physiology wire-based assessment for patients with stable chest pain and/or Ischemia and No Obstructive Coronary Artery Disease (INOCA).2,3
The AHA/ACC Clinical Practice Guideline3 on chest pain includes Class IIa recommendation for guidewire-based assessment for ischemia and no obstructed coronary artery disease (INOCA) patients.

The ESC guidelines2 2019 were updated accordingly to include an increased focus on microvascular dysfunction.

CFR = coronary flow reserve; CMR = cardiac magnetic resonance; ECG = electrocardiogram;
FFR = fractional flow reserve; iwFR = instantaneous wave-free ratio; LAD = left anterior descending; PET = position emission tomography.
a Class of recommendation
b Level of evidence
Level-NR: Level (Quality) of Evidence Level B-NR (non-randomized): moderate-quality evidence from 1 or more well-designed, well-executed non-randomized studies, observational studies or registry studies. RCTs. Meta-analyses of such studies.
An updated standardization of Coronary Microvascular Dysfunction (CMD) criteria is provided by the COVADIS group including the cut-off values of CFR < 2.0 and IMR ≥ 25 that characterize CMD.1
| Criteria | Evidence | Diagnostic Parameters |
|---|---|---|
| 1 | Symptoms of myocardial ischaemiaa |
|
| 2 | Absence of obstructive CAD (<50% diameter reduction or FFR >0.80) |
|
| 3 | Objective evidence of myocardial ischaemiab |
|
| 4 | Evidence of impaired coronary microvascular function |
|
Kunadian et al. European Heart Journal. 2020; 0:1-21.
Definitive microvascular angina is only diagnosed if criterias 1, 2, 3 and 4 are present.
CAD = coronary artery disease; CCTA = coronary computed tomographic angiography; CFR = coronary flow reserve; ECG = electrocardiogram; FFR = fractional flow reserve; IMR = index of microcirculatory resistance.
a Many patients with heart failure with preserved ejection fraction would fulfil these criteria: dyspnoea, no obstructive CAD and impaired CFR. For this reason, consider measuring LV end-diastolic pressure (normal ≤10 mmHg) and NT-proBNP normal <125 pg/mL.
b Signs of ischaemia may be present but are not necessary. However, evidence of impaired coronary microvascular function should be present.
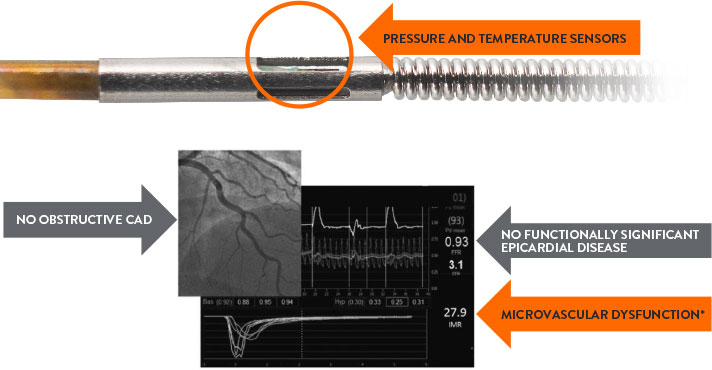
How to Diagnose CMD with a Comprehensive Physiology Assessment
The PressureWire™ X Guidewire and CoroFlow‡ Cardiovascular System is a comprehensive solution for assessing both epicardial arteries and the microvasculature.4
Diagnosis With Comprehensive Physiology Indices
Colin Berry MD, PhD, Glasgow, UK
The PressureWire™ X Guidewire has pressure and temperature sensors that communicate with the CoroFlow‡ Cardiovascular System to provide a comprehensive physiology assessment4
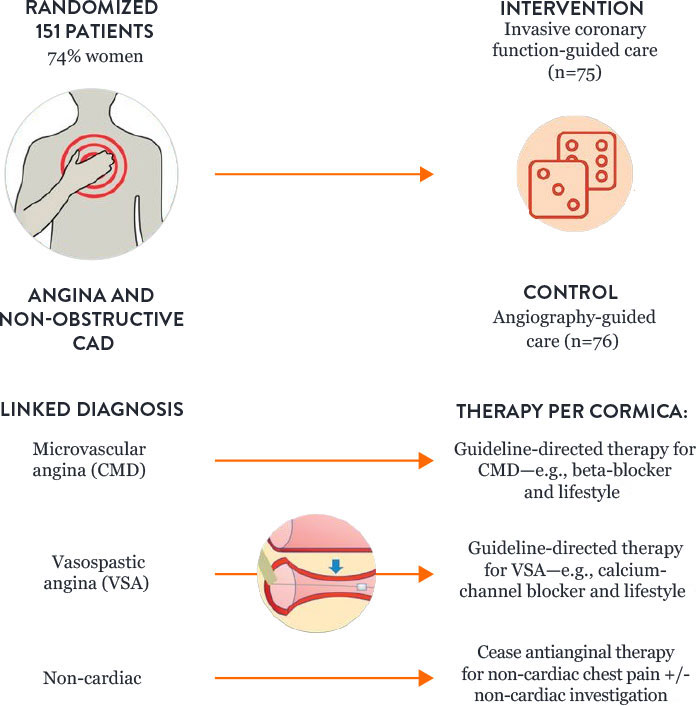
How to Treat Patients with CMD: Results of the CorMicA Trial
Microvascular angina and vasospastic angina are the two most common causes of INOCA, and both types of angina can be identified with diagnostic testing. The randomized CorMicA trial provides a diagnostic and treatment approach.5
The trial protocol assessed patients to determine:
- Microvascular angina or CMD—using the PressureWire™ X Guidewire
- Vasospastic angina—with acetylcholine testing
The CorMicA results indicate a role for a more thorough investigation of coronary microvascular dysfunction among patients with INOCA, as well as an opportunity to better tailor patient treatment.5
CorMicA Trial 1-year RCT Outcomes5
INOCA PATIENT MANAGEMENT
The EAPCI Expert Consensus Document1 defines INOCA and provides guidance to the clinical community on the diagnostic approach and management of INOCA based on existing evidence and best current practices.
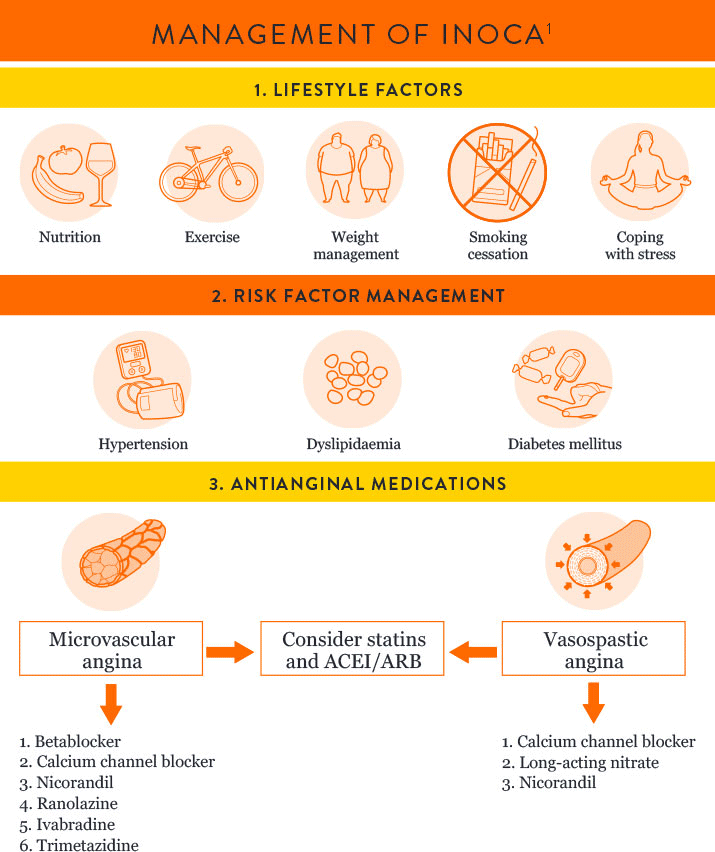
Angiotensin-converting enzyme inhibitor = ACEI
Angiotensin receptor blocker = ARB
References
- Kunadian, V., et al. EAPCI Expert Consensus. Eur Heart J 2020;0:1-21.
- Knuuti, J., et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. European Heart Journal 2020:41,407-477.
- Gulati, M., et al. 2021 AHA/ACC Guideline for the Evaluation and Diagnosis of Chest Pain. Circulation 2021;144e368-e454.
- PressureWire X Guidewire Instructions for Use (IFU). Refer to IFUs for additional information. CoroFlow Cardiovascular System IFU.
- Ford, TJ., et al. 1-year outcomes of angina management guided by invasive coronary function testing (CorMicA). J Am Coll Cardiol Intv. 2020;13:33-45.
MAT-2302513 v1.0
Important Safety Information
Coroventis‡ CoroFlow‡ Cardiovascular System

Indications: CoroFlow‡ is indicated to provide hemodynamic information for use in the diagnosis of patients with cardiovascular diseases.
CoroFlow‡ is intended for use in catheterization and related cardiovascular specialty laboratories to compute and display various physiological parameters based on the output from one or more measuring devices.
Contraindications: The system has no patient alarm functions. Do not use for cardiac/vital signs monitoring.
Warnings:
- If CoroFlow‡ is used together with 3rd party infusion catheters for assessment of Absolute Flow and Resistance, ensure that the maximum infusion rate per manufacturers instruction is not exceeded or vessel injury may occur.
- Do not use the CoroFlow‡ Cardiovascular System if there is reason to believe the system's security has been compromised or if the system was unaccounted for a period of time (i.e. misappropriated, modified or tampered with).
- Do not leave the CoroFlow‡ Cardiovascular System unattended when logged in as a PC Administrator.
- To protect the privacy and security of sensitive information, including electronic protected health information (EPHI), and to protect the integrity of the system itself, the system should be located in a physically secure, access-controlled environment.
- To protect the privacy and security of sensitive information, including electronic protected health information (EPHI), the PC on to which CoroFlow‡ is installed must be configured according to the Installation Instructions in this manual. Failure to configure the PC correctly may result in increased risk for unauthorized release of protected health information. Windows settings include:
- Activation and configuration of restricted user Access
- Activation of Windows Firewall and blocking of network connections
- Activation of Windows Bitlocker drive encryption
- Activation of Windows Secure Boot
- Activation of Windows Anti-Virus scanning and ransomware protection. Ensure CoroFlow‡ is added in the list of trusted applications.
- Activation of Windows update
- Disable unused interfaces
- Use of this equipment adjacent to or stacked with other equipment should be avoided because it could result in improper operation. If such use is necessary, this equipment and the other equipment should be observed to verify that they are operating normally.
- Use of accessories, transducers and cables other than those specified or provided by Coroventis‡ could result in increased electromagnetic emissions or decreased electromagnetic immunity of this equipment and result in improper operation.
- Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to any part of CoroFlow‡, including cables specified by Coroventis‡. Otherwise, degradation of the performance of this equipment could result.
Precautions:
- The PC and CoroHub‡ shall not be placed within the patient environment (1.5 m from patient).
- For operation of other devices used in conjunction with CoroFlow‡ consult the IFU for each of these devices for details on indication, handling and safety information.
- It is recommended to ensure local routines for data backup of stored recordings. CoroFlow‡ does not create backup of stored data.
- Always check minimum performance requirement on PC to ensure compatibility with CoroFlow‡.
- It is recommended to install CoroFlow‡ on a PC with backup battery to avoid interruption in case of power failure.
- Always manually review and confirm valid cursor positions and detected heart beats.
- Ensure that Pa and Pd pressure waveforms are aligned in phase and offset after equalization, or indices can be mis-calculated.
- Confirm that the correct Wi-Box is selected by manually matching the Wi-Box ID number with the Wi-Box in the lab.
- Changing parameter settings outside of default values may affect measurement performance, only for research purposes.
- Only to be used by healthcare professionals.
- Using a network location to store data may cause previously unidentified risks if the network malfunctions.
- No modification or tampering with CoroFlow‡ is permitted.
- CoroFlow‡, including accessories and components, shall not be used if it has been subject to damage.
- The assembly of medical electrical systems and modifications during the actual service life require evaluation to the requirements according to IEC 60601-1 standard series.
- CoroHub‡ does not have any serviceable parts and require no field maintenance. No modification or tampering with CoroHub‡ is permitted.
- CoroHub‡ shall not be immersed in liquid.
- CoroHub‡ shall not be used if it has been subject to damage.
- PPG values may be non-unique and different combinations or focal/diffuse disease may result in the same PPG value.
- Direct connection to a non-secure network, like the internet, may interfere with correct operation and/or result in inappropriate access to patient information. Furthermore, it should be noted that reconfiguring a used network may lead to inability to import patient as well as export examination data, ultimately leading to a risk of loss of patient and examination data. To avoid this problem Coroventis‡ recommends verifying network settings in the system setup after each change. The same caution is relevant regarding connection to DICOM.
- Always confirm valid pressure tracings, marker positions and selected beats.
- Resetting CoroHub‡ will reset PressureWire connections and Zeroing/Equalization parameters.
MAT-2007904 v4.0
PressureWire™ X Guidewire

INDICATIONS
The PressureWire™ X Guidewire is indicated to direct a catheter through a blood vessel and to measure physiological parameters in the heart and in the coronary and peripheral blood vessels. Physiological parameters include blood pressure. The PressureWire™ X Guidewire can also measure blood temperature.
CONTRAINDICATIONS
This guidewire is contraindicated for use in the cerebral vasculature.
POTENTIAL ADVERSE EVENTS
Potential adverse events which may be encountered during all catheterization procedures include, but are not limited to:
- Bleeding
- Hypotension / hypertension
- Chest pain
- Coronary / peripheral vascular injury
- Vascular dissection
- Perforation of vessels
- Stenosis
- Pseudoaneurysm
- Vasoconstriction
- Myocardial ischemia, tissue hypoxia
- Myocardial infarction
- Organ injury
- Thrombosis
- Death
- Arrhythmias
- Cardiac tamponade / pericardial effusion
- Bronchospasm (adenosine related)
- Prolonged procedure
- Foreign body in patient
- Urgent revascularization
- Congestive heart failure
- Embolus
- Local and / or systemic infection
- Device failures
- Hypersensitivity/ allergic reaction to device materials, medications or contrast
In addition, this device has a coating containing Polyethylene Glycol (PEG); potential allergic reactions (anaphylaxis) may occur during the interventional procedure of the patient is allergic to PEG.
WARNINGS
- The PressureWire™ X Guidewire must not be modified in any way by the customer. Making modifications may interfere with correct operation and will void product warranties. See your Abbott Technical Service representative for more information.
- Note the product “Use-by-date” on the package.
- The PressureWire™ X Guidewire is supplied sterile. Discard the guidewire if the pouch is opened or damaged, compromising the sterile barrier. The guidewire is designed for single use only and shall not be reused or resterilized. Adverse effects of using a non-sterile or resterilized guidewire may include, but are not limited to:
- Local and / or systemic infection
- Mechanical damage
- Inaccurate readings
- Observe all guidewire movements. Whenever the guidewire is moved or torqued, the tip movement should be examined under fluoroscopy. Never push, withdraw,
or torque the guidewire if it meets resistance or without observing corresponding movement of the tip; otherwise vessel/ventricle trauma may occur. - Torquing or excessive manipulation of the guidewire in a sharp bend, against resistance, or repeated attempts to cross a total vessel occlusion may:
- Cause dissection or perforation of blood vessels
- Cause vessel spasm
- Damage and / or fracture the guidewire
- When introducing the guidewire, flush the catheter and administer anticoagulation as for a standard catheterization procedure or clotting may occur.
- Do not use the guidewire in the ventricles if the patient has a prosthetic mechanical or biological valve. It may result in damage to both the prosthesis and the guidewire, which may cause injury or death.
- Use of the PressureWire™ X Guidewire in conjunction with interventional devices with a short rapid exchange may result in a folded or fractured guidewire.
- High frequency surgical devices must not be used on a patient at the same time as the guidewire.
- The safety and effectiveness of the coated device have not been established, or are unknown, in vascular regions other than those specifically indicated.
- Failure to abide by the warnings in this Instructions for Use might result in damage to the device coating, which may necessitate intervention or result in serious adverse events.
CAUTIONS
- The PressureWire™ X Guidewire is a delicate instrument and should be handled carefully.
- Make sure that the PressureWire™ X Guidewire transmitter is kept dry to ensure accurate pressure and/or temperature readings. Inaccurate readings or a wet transmitter may necessitate device replacement.
- Do not use the PressureWire™ X Guidewire in conjunction with atherectomy catheters. It may damage the PressureWire™ X Guidewire.
- Do not withdraw or manipulate the PressureWire™ X Guidewire with a sharp-edged object. It may result in abrasion of the PressureWire™ X Guidewire coating. When wet, the hydrophilic coating increases the lubricity of the coated surface.
- Factors that may affect the accuracy of the diagnostic information include, but are not limited to:
- Improper placement of the aortic pressure sensor.
- Failure to achieve maximum coronary and myocardial hyperemia in FFR procedures
- Blood flow affected by the position of interventional devices, such as balloon catheters.
- PressureWire™ X Guidewire readings may be affected by defibrillation. Rezero the guidewire after defibrillation use.
- Do not measure pressure when the PressureWire™ X Guidewire sensor element is in a sharp bend or in contact with atrial or ventricular walls. It might result in pressure artifacts.
- Do not use the PressureWire™ X Guidewire together with another guidewire, for so called jailed wire technique, due to difficulty in guidewire withdrawal and possible guidewire entrapment.
- Avoid abrasion of the distal hydrophilic and proximal PTFE coating regions. Use caution when manipulating, advancing and / or withdrawing these devices through needles, metal cannulas, stents, or other devices with sharp edges, or through tortuous or calcified blood vessels. Manipulation, advancement, and / or withdrawal past sharp or beveled edges may result in destruction and / or separation of the outer coating, which may lead to clinical adverse events, resulting in coating material remaining in the vasculature or device damage. This may result in adverse events requiring additional intervention.
- The integrity and performance of the device coating can be negatively impacted by preparation with incompatible media or solvents. Please take note of the following important recommendations:
- Avoid wiping the distal end of the device with dry gauze as this may damage the device coating; refer to Directions for Use for removal / reintroduction instructions.
- Avoid excessive wiping of the coated device.
- Avoid using alcohol, antiseptic solutions, or other solvents to pre-treat the device because this may cause unpredictable changes in the coating which could negatively affect the safety and performance of the device.
- Do not soak the device as it may adversely impact the hydrophilic coating on the device.
- The PressureWire™ X Guidewire contains parts with > 0.1% w/w concentration of 1-methyl-2-pyrrolidone or NMP, which has shown reproductive toxicity and teratogenicity in animal tests. This device has not been tested in pregnant women or in men intending to father children. Effects on the developing fetus have not been studied. In the worst-case scenario, the amount of NMP that may elute out of the PressureWire™ X Guidewire is > 25 times lower than the permissible daily exposure (PDE) by the ICH Q3C (R8) Guidelines for Residual Solvents. Occurrence of this worst-case scenario is highly unlikely, and the expected exposure time of the patient for the FFR procedure is 60 minutes. While there is no contraindication, the risks and reproductive effects of use of this device on humans are unknown at this time, It is recommended that the need of the patient and the clinical benefit of FFR be weighed against the risk stated above.
- One or more components of this device may contain the following substances defined as CMR 18 in a concentration above 0.1% weight by weight: Cobalt; Chemical Abstracts Service (CAS) No. 7440-48-4; EC No. 231-158-0. Current scientific evidence supports that medical devices manufactured from stainless steel alloys containing cobalt do not cause an increased risk of cancer or adverse reproductive effects.
- The safety and effectiveness of the PressureWire™ X Guidewire has not been established in the pediatric population.
- Persons with known history of allergies to any of the components of this device listed below may suffer an allergic reaction to this device. Prior to its use on the patient, the patient should be counseled on the material contained in the device, and a thorough history of allergies must be discussed. This device contains:
- Epoxy adhesive
- Gold
- Palladium-iridium alloy
- Polyethylene glycol
- Polyimide
- Polyvinyl pyrrolidone hydrophilic coating
- Polytetrafluoroethylene coating
- Silicone
- Silicon
- Silicon nitride
- Stainless steel
- Tin solder paste
- Store at room temperature (15°C to 25°C) in a dry and dark place.
MAT-2103599 v3.0
