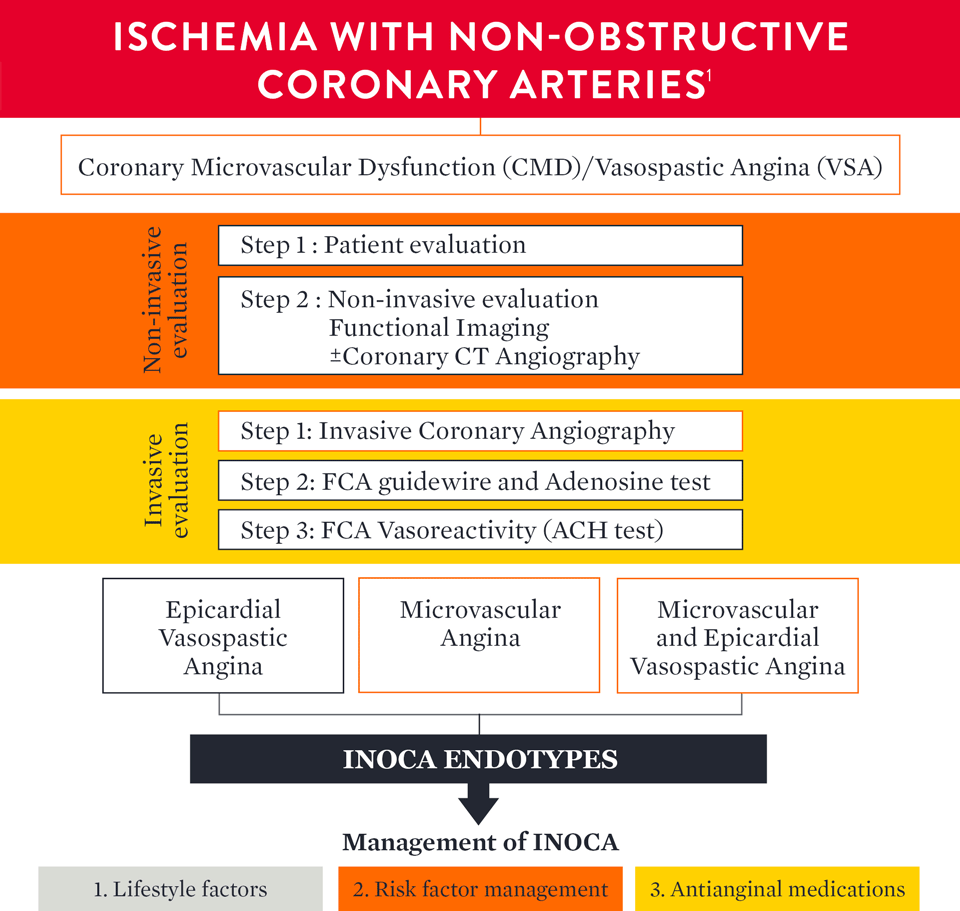
How to Diagnose INOCA
The European Association of Percutaneous Cardiovascular Interventions (EAPCI) Expert Consensus Document defines ischemia and no obstructive coronary artery disease (INOCA) and guidance to its diagnosis and management. The document states that, INOCA patients present with a wide spectrum of symptoms and signs that are often misdiagnosed as non-cardiac, leading to under-diagnosis/investigation and under-treatment.1
Guidelines Recommend Guidewire-Based Measurements
According to the ESC 2024 guidelines for Chronic Coronary Syndromes, invasive coronary angiography, along with invasive functional testing (assessing for IMR and CFR) is a Class 1B recommendation and is recommended to confirm or exclude the diagnosis of obstructive CAD or INOCA2
| Recommendation | Classa | Levelb |
|---|---|---|
| Invasive coronary angiography with the availability of invasive functional assessment is recommended to confirm or exclude the diagnosis of obstructive CAD or ANOCA/INOCA in individuals with an uncertain diagnosis on non-invasive testing. | I | B |
IMR = index of microcirculatory resistance; CFR = coronary flow reserve; CAD = coronary artery disease.
a Class of recommendation
b Level of evidence
An updated standardization of Coronary Microvascular Dysfunction (CMD) criteria is provided by the COVADIS group including the cut-off values of CFR ≥ 2.53,4 and
IMR ≥ 25 that characterize CMD.1
| Criteria | Evidence | Diagnostic Parameters |
|---|---|---|
| 1 | Symptoms of myocardial ischaemiaa |
|
| 2 | Absence of obstructive CAD (<50% diameter reduction or FFR >0.80) |
|
| 3 | Objective evidence of myocardial ischaemiab |
|
| 4 | Evidence of impaired coronary microvascular function |
|
Definitive microvascular angina is only diagnosed if criterias 1, 2, 3 and 4 are present.
CAD = coronary artery disease; CCTA = coronary computed tomographic angiography; CFR = coronary flow reserve; ECG = electrocardiogram; FFR = fractional flow reserve; IMR = index of microcirculatory resistance.
a Many patients with heart failure with preserved ejection fraction would fulfil these criteria: dyspnoea, no obstructive CAD and impaired CFR. For this reason, consider measuring LV end-diastolic pressure (normal ≤10 mmHg) and NT-proBNP normal <125 pg/mL.
b Signs of ischaemia may be present but are not necessary. However, evidence of impaired coronary microvascular function should be present.
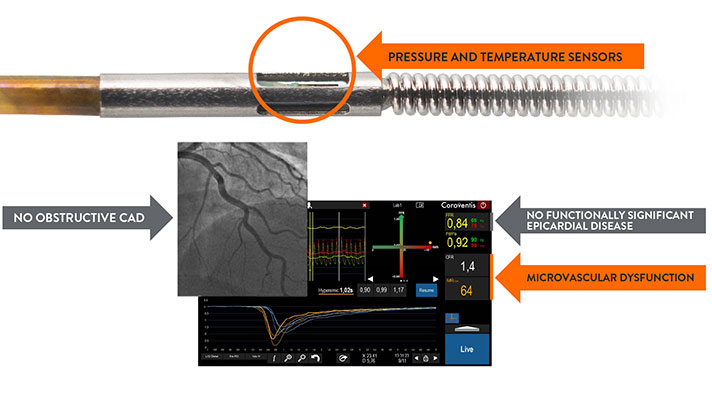
How to Diagnose CMD with a Full Physiology Assessment
The PressureWire™ X Guidewire has pressure and temperature sensors that communicate with the CoroFlow‡ Cardiovascular System to conduct a full physiology assessment of epicardial arteries and microvasculature.5
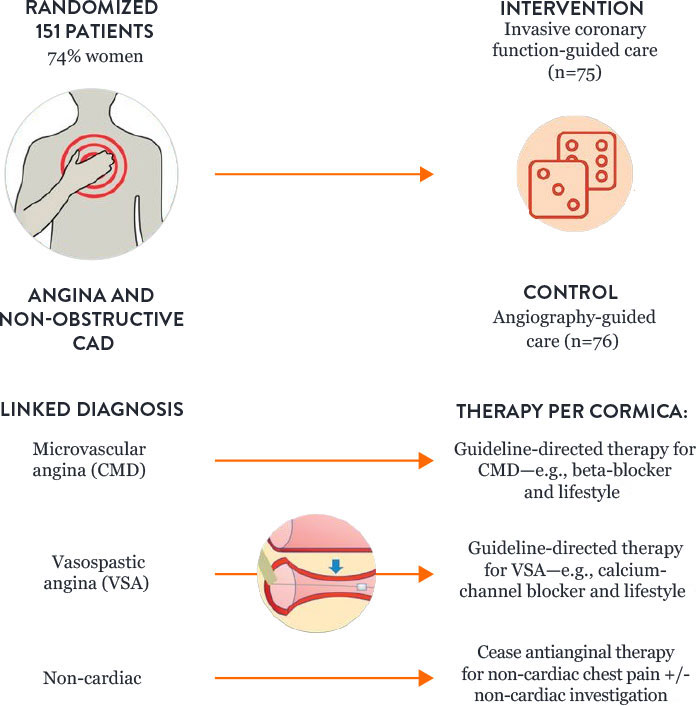
How to Treat Patients with CMD: Results of the CorMicA Trial
Microvascular angina and vasospastic angina are the two most common causes of INOCA, and both types of angina can be identified with diagnostic testing. The randomized CorMicA trial provides a diagnostic and treatment approach.4
The trial protocol assessed patients to determine:
- Microvascular angina or CMD—using guidewire-based assessment
- Vasospastic angina—with acetylcholine testing
The CorMicA results indicate a role for a more thorough investigation of coronary microvascular dysfunction among patients with INOCA, as well as an opportunity to better tailor patient treatment.4
CorMicA Trial 1-year RCT Outcomes4
INOCA PATIENT MANAGEMENT
The EAPCI Expert Consensus Document1 defines INOCA and provides guidance to the clinical community on the diagnostic approach and management of INOCA based on existing evidence and best current practices.
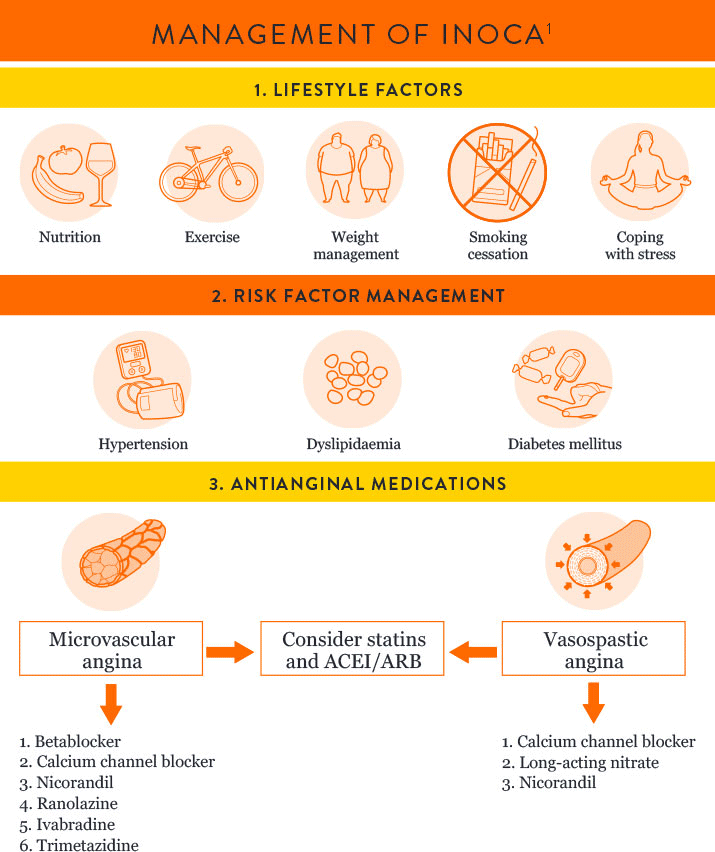
Angiotensin-converting enzyme inhibitor = ACEI
Angiotensin receptor blocker = ARB
References
- Kunadian, V., et al. EAPCI Expert Consensus. Eur Heart J 2020;0:1-21.
- Vrints C., et al. 2024 ESC guidelines for the management of complex coronary syndromes. Eur Heart J. 2024;45(36):3415-3537.
- Kunadian V., et al. EAPCI Expert Consensus Document. EuroIntervention 2021;16:1049-1069.
- Ford, TJ., et al. 1-year outcomes of angina management guided by invasive coronary function testing (CorMicA). J Am Coll Cardiol Intv. 2020;13:33-45.
- PressureWire™ X Guidewire Instructions for Use (IFU). Coroflow‡ Cardiovascular System (IFU). Refer to IFUs for additional information.
MAT-2112674 v3.0
