Capture with Confidence
Indicated for Carotids and Lower Extremities
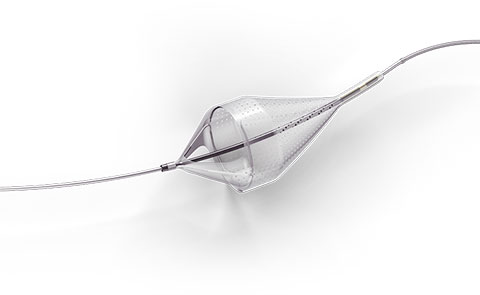
The Emboshield NAV6™ Embolic Protection System, which includes BareWire™ Filter Delivery Wires, allows the guide wire to rotate and advance freely, independent of the Emboshield NAV6™ filter.*
The Emboshield NAV6™ EPS is a temporary percutaneous transluminal filtration system designed to be used as a guide wire and to capture embolic material released during an interventional procedure within the carotid artery or the arterial vasculature of the lower extremity.
Embolic Protection Device for Both Lower Extremity and Carotid Arteries
Captures Effectively to Minimize Downstream Complications1-3
- Centered wire design prevents bias against the vessel wall for effective debris capture.*
- Circumferential nitinol frame maintains optimal wall apposition, even on a bend.*
- Platinum-tungsten frame coils provide excellent visibility.*
- Two sizes allow for easy selection and minimal lab inventory.
Limits Filter Movement and Maintains Wire Access Through Innovative BareWire™ Design*
- The unique BareWire™ technology allows the wire to rotate and advance freely, independently of the filter.
- The filter is designed to stay in place during device delivery.*
- Continued wire access, after filter is fully retracted, allows for easy delivery of additional therapy.*
Navigates Skillfully Through Carotid and Lower Extremity Vasculature*
- Various types of BareWires™—distal access, workhorse, and support—are designed for various carotid anatomies.
- They promote navigational success through torturous anatomy and challenging arches.
Is Compatible with Various Atherectomy Options4
- Emboshield NAV6™ EPS and BareWire™ are compatible with a variety of atherectomy types.
- BareWire™ is available in 190 cm and 315 cm lengths.
In carotid procedures, the Emboshield NAV6™ EPS is used during stenting with the RX Acculink™ Carotid Stent System and the Xact™ Carotid Stent System.
* Data on file at Abbott.
References
- Bioangiu et al. Comparative analysis of retrieved particulate debris after superficial femoral atherectomy using three different atherectomy methods. EuroIntervention, May 2012.
- Bioangiu et al. Analysis of Retrieved Particulate Debris after Superficial Femoral Artery (SFA) Atherectomy Using the Pathway Jetstream G3 device. CCI, May 2011, 77(2) p. S57.
- Mendes et al. Clinical significance of embolic events in patients undergoing endovascular femoropopliteal interventions with or without embolic protection devices. JVS, February 2014, 59(2), 359-367.
- Philips Turbo-Elite, Medtronic TurboHawk PPES, and Boston Scientific Jetstream XC and SC. Data on file at Abbott.
MAT-2116946 v2.0
