Accuracy Where it Counts
Exact placement, strength and performance where it counts.
Best In-Class Product1
- More than 10,000 patients have received an Abbott XACT™ Carotid Stent across 5 clinical studies and registries
- Proven Low Stroke Rates
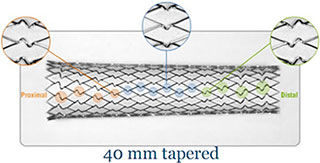
Unique Stent Design
- Variable cell design provides targeted radial strength and offers strength suited to anatomy and lesion location and tailers coverage in targeted vessel segments
- Straight and tapered stent configurations
- Closed-cell design for crush resistance and minimized plaque extrusion
Easy Deployment
- Ergonomic handle offers single-handed deployment for controlled, smooth stent deployment
- Rotational deployment handle with Freestyle™ technology stabilizer for controlled, accurate stent deployment
- Stabilizer absorbs frictional forces that may cause inaccurate stent deployment
Unique Variable Cell Size
- Increased radial force at lesion
- Increased stent coverage at lesion designed to minimize plaque extrusion
- Cell design minimizes risk of snagging

Cell Geometry
- U- and S-shaped connectors affect cell geometry, and combined with cell length, affect radial strength and cell area
- Benefits of S-shaped connectors include increased stent flexibility for improved delivery, opportunity to allow the stent to open more fully and the increased cell length reduces the metal-to artery ratio in healthy arterial segments
- Benefits of flared ends include stable stent deployment and decreases the potential for retrieval catheter or filter snagging
Data on file at Abbott.
Ordering Information
| XACT™ Carotid Stent System (FDA Part Numbers) | ||||||
|---|---|---|---|---|---|---|
| Stent Diameter (mm) | Stock Number | ICA Reference Vessel Diameter (mm) | CCA Reference Vessel Diameter (mm) | Minimum Sheath / Guide Size | ||
| 20 (mm) | 30 (mm) | 40 (mm) | ||||
| 7.0 | 82095-01 | 82094-01 | N/A | 5.5-6.4 | N/A | 6F / 8F |
| 8.0 | 82093-01 | 82092-01 | N/A | 6.4-7.3 | N/A | 6F / 8F |
| 9.0 | 82089-01 | 82088-01 | N/A | 7.3-8.2 | N/A | 6F / 8F |
| 10.0 | 82099-01 | 82098-01 | N/A | 8.2-9.1 | N/A | 6F / 8F |
| 6.0-8.0 taper | N/A | 82091-01 | 82090-01 | 4.8-5.5 | 6.4-7.3 | 6F / 8F |
| 7.0-9.0 taper | N/A | 82087-01 | 82086-01 | 5.5-6.4 | 7.3-8.2 | 6F / 8F |
| 8.0-10.0 taper | N/A | 82097-01 | 82096-01 | 6.4-7.3 | 8.2-9.1 | 6F / 8F |
| X.ACT™ Carotid Stent System (CE Part Numbers) | ||||||
|---|---|---|---|---|---|---|
| Stent Diameter (mm) | Stock Number | ICA Reference Vessel Diameter (mm) | CCA Reference Vessel Diameter (mm) | Minimum Sheath / Guide Size | ||
| 20 (mm) | 30 (mm) | 40 (mm) | ||||
| 7.0 | XRX02007S | XRX03007S | N/A | 5.5-6.4 | N/A | 6F / 8F |
| 8.0 | XRX02008S | XRX03008S | N/A | 6.4-7.3 | N/A | 6F / 8F |
| 9.0 | XRX02009S | XRX03009S | N/A | 7.3-8.2 | N/A | 6F / 8F |
| 10.0 | XRX02010S | XRX03010S | N/A | 8.2-9.1 | N/A | 6F / 8F |
| 6.0-8.0 taper | N/A | XRX03008T | XRX04008T | 4.8-5.5 | 6.4-7.3 | 6F / 8F |
| 7.0-9.0 taper | N/A | XRX03009T | XRX04009T | 5.5-6.4 | 7.3-8.2 | 6F / 8F |
| 8.0-10.0 taper | N/A | XRX03010T | XRX04010T | 6.4-7.3 | 8.2-9.1 | 6F / 8F |
Data on file at Abbott.
References
- Lal, B. et al. Quality Assurance for Carotid Stenting in the CREST-2 Registry. Vol. 74 No. 25. 2019
MAT-2116955 v2.0
