Excellent Outcomes:
Emboshield NAV6TM EPS Clinical Data
Embolic Protection Devices can add a layer of protection during endovascular procedures in the lower extremities or carotids. In the lower extremities, downstream complications can be minimized and in the carotids, the stroke rate can be reduced with the use of EPDs.1
Clinical Findings in Brief
Three trials evaluated carotid stenting with the Emboshield NAV6™ EPS and/or previous generation Abbott products.
| Summary of Clinical Trial Data for Emboshield NAV6™ Embolic Protection System | |||
|---|---|---|---|
| ACT I2 (n = 1,453; carotid stenting n = 1,089) Asymptomatic patients at standard risk for CEA | CHOICE3 (n = 17,925) Symptomatic and asymptomatic patients at high risk for CEA | PROTECT4 (n = 220) Symptomatic and asymptomatic patients at high risk for CEA | |
| DSMI (30 Days) | 3.3% | 4.2% | 2.3% |
| DS (30 Days) | 2.9% | 3.8% | 1.8% |
| Death or Major Stroke (30 Days) | 0.6% | 1.4% | 0.5% |
| Freedom from Ipsilateral Stroke | 97.8% (30 Days – 5 Years) | Not evaluated | 98.8% (31 Days – 3 Years) |
DS = Death or stroke | DSMI = Death, stroke, or myocardial infarction | CEA = Carotid endarterectomy
ACT I Trial2,5
The primary aim of this prospective, multicenter trial was to compare the outcomes of stenting with embolic protection vs carotid endarterectomy.
Patients2
The 1,453 patients were randomly assigned to the stenting group (n = 1,089) or the CEA group (n = 364), all of whom met the following criteria:
- Asymptomatic, severe (> 70%) carotid stenosis
- ≤ 79 years of age
- Standard risk for CEA surgical complications
Findings2
| ACT I Trial | ||
|---|---|---|
| Carotid Artery Stenting (CAS) | Carotid Endarterectomy | |
| Primary composite endpoint: DSMI (at 30 days) and ipsilateral stroke (31 days - 1 year) | 3.8% | 3.4% |
| Freedom from ipsilateral stroke (31 days - 5 years) | 97.8% | 97.3% |
| Freedom from clinically driven revascularization (5 years, p = 0.05) | 98.4% | 96.7% |
| 5 year survival | 87.1% | 89.4% |
The authors concluded that:
- There were no significant differences in long-term (5-year) rates of stroke and survival between the two groups in this large randomized trial.5
- CAS was found to be noninferior to CEA for the primary composite end point: DSMI within 30 days; or ipsilateral stroke within 1 year post procedure.5
CHOICE Trial3
With 17,925 patients evaluated, the CHOICE trial represents the largest prospective, single-arm, adjudicated, multicenter CAS data set to date. The CHOICE study also provided additional post-market surveillance of RX Acculink™ Carotid Stent System and Abbott’s embolic protection systems.
Patients
Patient criteria included:
- Severe stenosis of ≥ 50% for symptomatic patients and ≥ 80% for asymptomatic patients
- High surgical risk for CEA
There were other notable aspects of the patient population:
- 22.6% who were age ≥ 80
- 24.4% who had heavy calcification at the target site
Findings
The 30-day findings included:
| CHOICE Trial | ||
|---|---|---|
| All Patients (n = 17,925) | Patients Age < 80 (n = 13,868) | |
| DSMI | 4.2% | 3.4% |
| DS | 3.8% | 3.0% |
| Death or major stroke | 1.4% | 1.1% |
The investigators concluded that CAS is a viable option for patients at high risk for CEA. In addition, favorable outcomes were observed in patients < 80 years of age.
PROTECT Trial4
Investigators undertook the PROTECT trial (n = 220) in an effort to evaluate the outcomes with improved device technology.
Patients
The PROTECT trial included only patients at high surgical risk for CEA, and severe stenosis:
- ≥ 50% for symptomatic patients
- ≥ 80% for asymptomatic patients
Findings
| PROTECT Trial | |
|---|---|
| DS (30 days) | 1.8% |
| DSMI (30 days) | 2.3% |
| Death or Major Stroke (30 days) | 0.5% |
| Freedom from Ipsilateral Stroke (31 days – 3 years) | 98.8% |
These data reveal improved outcomes compared to earlier high-risk CAS trials.
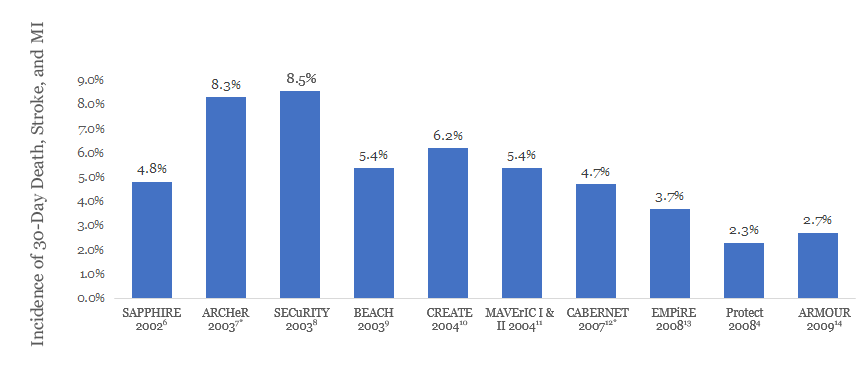
NOTE: Results from clinical trials are not directly comparable. Information provided for educational purposes only.
Lower Extremity Real World Data Analysis15
The Lower Extremity Real World Data Analysis is a prospective analysis of real-world data collected from 162 patients receiving embolic protection with Emboshield NAV6™ during atherectomy for femoral popliteal lesions in real-world clinical practice.
The primary analysis outcome was freedom from major adverse events (MAE) at 30 days, which was compared against a performance goal derived from MAE rates of similar devices used in the same anatomy.
Patients
Patient criteria:
- Patients with symptomatic lower extremity PAD, which includes femoropopliteal lesions above the P2 segment and all TASC classifications, who received atherectomy in conjunction with Emboshield NAV6™ at the Mount Sinai Health Center between January 2014 and October 2015. Patients with critical limb ischemia or inflow disease, including the iliac, were excluded
Patients were treated per standard of care and choice of atherectomy, atherectomy device, use of filter, and filter type was at the discretion of the operator.
Findings
All Emboshield NAV6™ filters were delivered successfully, and there were no reports of device malfunction. One complication, a perforation caused by migration of the wire in the filter, occurred. The complication was treated without sequelae. Macroemboli was present in approximately 60% of cases, and filter overflow occurred in 10.5% of cases.
The 30-day freedom from MAE rate was 92.0%, in which the lower limit of the two-sided 95% confidence interval was 86.7%, meeting the pre-specified PG of 83%.
| Freedom from Major Adverse Events Rate vs. Performance Goal | ||
|---|---|---|
| Primary Endpoint | Performance Goal | Emboshield NAV6™ EPS (n = 162) |
| Freedom from MAE [95% Confidence Interval]1 | 83% | 92.0% (149/162) [86.7%, 95.7%] |
References
- Banerjee A, Sarode K, Mohammad A, et al. Safety and Effectiveness of the Nav-6 Filter in Preventing Distal Embolization During Jetstream Atherectomy of Infrainguinal Peripheral Artery Lesions. J Invasive Cardiol. 2016;28(8):330-333. Kastrup, et al. Early Outcome of Carotid Angioplasty and Stenting With and Without Cerebral Protection Devices: A Systematic Review of the Literature. Stroke, Mar 2003, 34(3):813-9. Mendes et al. Clinical significance of embolic events in patients undergoing endovascular femoropopliteal interventions with or without embolic protection devices. JVS, February 2014, 59(2), 359-367.
- Rosenfield K, Matsumura JS, Chaturvedi S, et al. Randomized trial of stent versus surgery for asymptomatic carotid stenosis. N Engl J Med. 2016;374(11):1011-1120.
- Metzger DC. The CHOICE prospective trial: carotid stenting in a post-market setting. VIVA 2013.
- Matsumura JS, Gray W, Chaturvedi S, et al. Results of carotid artery stenting with distal embolic protection with improved systems: Protected Carotid Artery Stenting in Patients at High Risk for Carotid Endarterectomy (PROTECT) trial. J Vasc Surg. 2012; 55(4):968-976.e5.
- Wechsler, LR. Asymptomatic Carotid Stenosis Stenting v. Endarterectomy Trial (ACT I). ISC 2016.
- Yadav et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493-501.
- Gray et al. Protected carotid stenting in high-surgical-risk patients: The ARCHeR results. JVS 2006;44(2) 258-68.
- U.S. XACT™ Carotid Stent System - Instructions for Use (IFU)
- Iyer et al. Carotid artery revascularization in high-surgical-risk patients using the Carotid WALLSTENT and FilterWire EX/EZ: 1-year outcomes in the BEACH Pivotal Group. J Am Coll Cardiol. 2008 Jan 29;51(4):427-34
- Safian et al. Protected carotid stenting in high-risk patients with severe carotid artery stenosis. J Am Coll Cardiol. 2006;47:2384-2389.
- Higashida et al. Evaluation of the Medtronic Exponent Self-Expanding Carotid Stent System With the Medtronic Guardwire Temporary Occlusion and Aspiration System in the Treatment of Carotid Stenosis. Stroke. 2010 Feb;41(2):e102-9.
- Hopkins et al. Carotid artery revascularisation in high-surgical-risk patients with the NexStent and the FilterWire EX/EZ: 3-year results from the CABERNET trial. EuroIntervention. 2010;5:917-924.
- Clair et al. Neuroprotection during carotid artery stenting using the GORE flow reversal system: 30-day outcomes in the EMPiRE Clinical Study. Catheter Cardiovasc Interv. 2011 Feb 15;77(3):420-9.
- Ansel et al. Safety and effectiveness of the INVATEC MO.MA proximal cerebral protection device during carotid artery stenting: results from the ARMOUR pivotal trial. Catheter Cardiovasc Interv. 2010;76:1-8.
- U.S. Emboshield NAV6TM Embolic Protection System Instructions for Use (IFU). Refer to IFU for additional information.
* 30-day death, stroke, and MI plus late (31–365 days) ipsilateral stroke
MAT-2200415 v2.0
