At Abbott, we are committed to advancing innovative technologies that improve the lives of people living with structural heart diseases. We strive to provide safe, effective, and minimally invasive treatment options that address the unique needs of these patients. We led the way in Transcatheter Edge-to-Edge Repair (TEER) with the development of the MitraClip™ and TriClip™ TEER Systems, offering transformative solutions for patients suffering from severe mitral regurgitation (MR) and symptomatic severe tricuspid regurgitation (TR). Building on the legacy of innovation, we reimagined the treatment of aortic stenosis with the Navitor™ TAVI System. Together, these innovations reflect Abbott’s leadership in structural heart therapies, empowering clinicians and transforming care for patients around the world.
**TAVI is also referred to as TAVR (Transcatheter Aortic Valve Replacement)
MitraClip™ TEER Therapy
MitraClip™ Transcatheter Edge-to-Edge Repair (TEER) procedure is a minimally invasive treatment option, uniquely designed for the mitral valve, for select patients with primary or secondary MR. MitraClip™ TEER clinical evidence set the foundation for TEER recommendations from ACC/AHA,1 ESC/EACTS2-3 and ASPC4. As the standard of care in TMVr built on the only proven mitral valve therapy with over 20+ years of experience, extensive body of clinical evidence, and more than 200,000 patients treated worldwide,5 allowing physicians to treat more patients with more options.
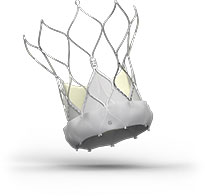
Navitor™ TAVI System
Advancing the forefront of innovative design, the Navitor™ Transcatheter Aortic Valve Implantation (TAVI) System brings together stable delivery, remarkable performance, and a future-ready design.
TriClip™ TEER System
TriClip™ Transcatheter Edge-to-Edge Repair (TEER) is a non-surgical treatment option for patients with symptomatic severe Tricuspid Regurgitation (TR), despite optimal medical therapy. TriClip TEER offers exceptional safety7,8 and maximized effectiveness9,10 to significantly improve quality of life (QoL) and functional status of patients with TR.11
References
- Heidenreich PA, et al. "2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association. Joint Committee on Clinical Practice Guidelines." J Am Coll Cardiol. Apr 01,2022. Epublished DOI: 10.1016/j.jacc.2021.12.012.
- Praz F, Borger M, Lanz J, et al., ESC/EACTS Scientific Document Group, 2025 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS), European Heart Journal, 2025; ehaf194, https://doi.org/10.1093/eurheartj/ehaf194.
- McDonagh TA, Metra M, Adamo M, et al., ESC Scientific Document Group, 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC, European Heart Journal, Volume 42, Issue 36, 21 September 2021, Pages 3599–3726, https://doi.org/10.1093/eurheartj/ehab368.
- Yeo KK, Tan JWC, Muller DW, et al. Asian Pacific Society of Cardiology Consensus Recommendations on the Use of MitraClip for Mitral Regurgitation. Eur Cardiol. 2021;16:e25. Published 2021 Jun 1. doi:10.15420/ecr.2021.01.
- Data on File at Abbott.
- Navitor™ TAVI System IFU.
- Tang GHL, Hahn RT, Whisenant BK, et al. Tricuspid transcatheter edge-to-edge repair for severe tricuspid regurgitation: 1-year outcomes from the TRILUMINATE randomized cohort. J Am Coll Cardiol. Published online 2025;85(3):235-246. doi:10.1016/j.jacc.2024.10.08.
- Lurz P, Rommel KP, Schmitz T, et al. Real-world 1-year results of tricuspid edge-to-edge repair from the bRIGHT study. J Am Coll Cardiol. 2024;84(7):607-616. doi:10.1016/j.jacc.2024.05.006.
- Kar S, Makkar RR, Whisenant BK, et al. Two-year outcomes of transcatheter edge-to-edge repair for severe tricuspid regurgitation: The TRILUMINATE Pivotal Randomized Trial. Circulation. March 30, 2025. doi:10.1161/CIRCULATIONAHA.125.074536.
- Estevez-Loureiro R. Real-world Outcomes for Tricuspid Edge-to-Edge Repair: Initial 2-year Outcomes from the bRIGHT Trial. Presented at PCR London Valves on November 19-21, 2023; London, UK.
- Nickenig G. Percutaneous edge-to-edge repair for tricuspid regurgitation: 3-year outcomes from the TRILUMINATE trial. Presented at PCR London Valves on November 19-21, 2023; London, UK.
MAT-2011953 v8.0
Rx Only
Important Safety Information
MITRACLIP™ CLIP DELIVERY SYSTEM
Indications for Use
The MitraClip™ G5 System is indicated for the percutaneous reduction of significant symptomatic mitral regurgitation (MR ≥ 3+) due to primary abnormality of the mitral apparatus [degenerative MR] in patients who have been determined to be at prohibitive risk for mitral valve surgery by a heart team, which includes a cardiac surgeon experienced in mitral valve surgery and a cardiologist experienced in mitral valve disease, and in whom existing comorbidities would not preclude the expected benefit from reduction of the mitral regurgitation.
The MitraClip™ G5 System, when used with maximally tolerated guideline-directed medical therapy (GDMT), is indicated for the treatment of symptomatic, moderate-to-severe or severe secondary (or functional) mitral regurgitation (MR; MR ≥ Grade III per American Society of Echocardiography criteria) in patients with a left ventricular ejection fraction (LVEF) ≥ 20% and ≤ 50%, and a left ventricular end systolic dimension (LVESD) ≤ 70 mm whose symptoms and MR severity persist despite maximally tolerated GDMT as determined by a multidisciplinary heart team experienced in the evaluation and treatment of heart failure and mitral valve disease.
Contraindications
The MitraClip™ G5 System is contraindicated for use in patients with the following conditions:
- Intolerance, including allergy or untreatable hypersensitivity to, procedural anticoagulation
- Untreatable hypersensitivity to Implant components (nickel- titanium alloy, cobalt-chromium alloy)
- Active endocarditis or other active infection of the mitral valve
Potential Complications and Adverse Events
The following events have been identified as possible complications of the MitraClip™ G5 procedure:Allergic reactions or hypersensitivity to latex, contrast agent, anaesthesia, device materials, and drug reactions to anticoagulation, or antiplatelet drugs, additional treatment/surgery from device-related complications, atrial septal defect, bleeding, blood disorders (including coagulopathy, hemolysis, and Heparin Induced Thrombocytopenia (HIT)), Cardiac arrhythmias (including conduction disorders, atrial arrhythmias, ventriculararrhythmias), Cardiac ischemic conditions (including myocardial infarction, myocardial ischemia, unstable angina, and stable angina), Cardiac perforation, Cardiac tamponade, chest pain, death, Dyspnea, Edema, Embolization (device or components of the device), Endocarditis, fever or hyperthermia, Fluoroscopy and Transesophageal echocardiogram (TEE) -related complications: Skin injury or tissue changes due to exposure to ionizing radiation, Esophageal irritation, Esophageal perforation, Gastrointestinal bleeding, Hypotension / hypertension; Infection including: Septicemia; Mitral valve complications, which may complicate or prevent later surgical repair, including: Chordal entanglement / rupture, Single leaflet device attachment (SLDA), dislodgement of previously implanted devices, tissue damage, mitral valve stenosis, worsening, persistent or residual regurgitation, nausea or vomiting, pain, Pericardial effusion, stroke / cerebrovascular accident (CVA) and transient ischemic attack (TIA), system organ failure: cardio-respiratory arrest, worsening heart failure, Pulmonary congestion, Respiratory dysfunction or failure or atelectasis, Renal insufficiency or failure, Shock (including cardiogenic and anaphylactic), Thrombosis, Vascular access complications which may require additional intervention, including: wound dehiscence, bleeding of the access site, Arteriovenous fistula, pseudoaneurysm, aneurysm, dissection, perforation (rupture), vascularocclusion, Embolism (air, thrombus), Peripheral nerve injury, Venous thrombosis (including deep vein thrombosis) and thromboembolism (including pulmonary embolism)
Rx Only
Important Safety Information
NAVITORTM TRANSCATHETER AORTIC VALVE IMPLANTATION SYSTEM
Indications
The Navitor™ Transcatheter Aortic Valve Implantation System is indicated for relief of aortic stenosis in patients with symptomatic heart disease due to severe native calcific aortic stenosis who are judged by a heart team, including a cardiac surgeon, to be high or greater risk for open surgical therapy (i.e., predicted risk of surgical mortality ≥ 8% at 30 days, based on the Society of Thoracic Surgeons (STS) risk score and other clinical comorbidities unmeasured by the STS risk calculator).
Contraindications
The valve is contraindicated for patients with inability to tolerate antiplatelet/anticoagulant therapy or nitinol alloy (nickel and titanium), or who have active infections, including endocarditis.
Potential Adverse Events
Adverse events potentially associated with the use of transcatheter bioprosthetic heart valves include but are not limited to: access site complications (e.g., pain, bleeding, infection, hematoma, pseudoaneurysm, etc.); acute coronary obstruction; acute myocardial infarction; allergic reaction to antiplatelet agents, contrast medium, or valve components; aortic rupture; ascending aorta trauma; atrio-ventricular node block; cardiac arrhythmias; conduction system injury; conversion to open surgical procedure; death; dissection; embolism; emergent balloon valvuloplasty; emergent percutaneous coronary intervention (PCI); emergent surgery (i.e., coronary artery bypass, heart valve replacement); endocarditis; explantation; heart failure; hemodynamic compromise; hemolysis; hemolytic anemia; hemorrhage; hypotension or hypertension; infection; myocardial ischemia; mitral valve insufficiency; multi-organ failure; non-structural dysfunction (i.e., entrapment by pannus, paravalvular leak, inappropriate sizing or positioning); pannus; pericardial effusion; perforation of the myocardium, ventricle, or a blood vessel; permanent disability; permanent pacemaker; regurgitation; renal insufficiency or renal failure; reoperation; respiratory failure; sepsis; stroke; structural deterioration (i.e., calcification, leaflet tear); thrombosis; tamponade; transfusion; valve embolization or migration; vessel dissection or spasm.
Rx Only
Important Safety Information
TRICLIP™ CLIP G5 SYSTEM
INDICATIONS
The TriClip™ G5 System is indicated for improving quality of life and functional status in patients with symptomatic severe tricuspid regurgitation despite optimal medical therapy, who are at intermediate or greater risk for surgery and in whom transcatheter edge-to-edge valve repair is clinically appropriate and is expected to reduce tricuspid regurgitation severity to moderate or less, as determined by a multidisciplinary heart team.
CONTRAINDICATIONS
The TriClip™ G5 System is contraindicated for use in patients with the following conditions: Intolerance, including allergy or untreatable hypersensitivity, to procedural anticoagulation; Untreatable hypersensitivity to Implant components (nickel-titanium alloy, cobalt-chromium alloy); Active endocarditis or other active infection of the tricuspid valve.
POTENTIAL ADVERSE EVENTS
The following events have been identified as possible complications of the TriClip™ G5 Procedure. Allergic reactions or hypersensitivity to latex, contrast agent, anaesthesia, device materials and drug reactions to anticoagulation, or antiplatelet drugs; Additional treatment / surgery from device-related complications; Bleeding; Blood disorders (including coagulopathy, hemolysis, and Heparin Induced Thrombocytopenia (HIT)); Cardiac arrhythmias (including conduction disorders, atrial arrhythmias, ventricular arrhythmias); Cardiac ischemic conditions (including myocardial infarction, myocardial ischemia, unstable angina, and stable angina); Cardiac perforation; Cardiac tamponade; Chest pain; Death; Dyspnea; Edema; Embolization (device or components of the device); Endocarditis; Fever or hyperthermia; Fluoroscopy and Transesophageal echocardiogram (TEE) -related complications: Skin injury or tissue changes due to exposure to ionizing radiation, Esophageal irritation, Esophageal perforation, Gastrointestinal bleeding; Hypotension / hypertension; Infection including: Septicemia; Nausea or vomiting; Pain; Pericardial effusion; Stroke / cerebrovascular accident (CVA) and transient ischemic attack (TIA); System organ failure: Cardio-respiratory arrest, Worsening heart failure, Pulmonary congestion, Respiratory dysfunction or failure or atelectasis, Renal insufficiency or failure, Shock (including cardiogenic and anaphylactic); Thrombosis; Tricuspid valve complications, which may complicate or prevent later surgical repair, including: Chordal entanglement / rupture, Single leaflet device attachment (SLDA), Dislodgement of previously implanted devices, Tissue damage, Tricuspid valve stenosis, Worsening, persistent or residual regurgitation; Vascular access complications which may require additional intervention, including: Wound dehiscence, Bleeding of the access site, Arteriovenous fistula, pseudoaneurysm, aneurysm, dissection, perforation (rupture), vascular occlusion, Embolism (air, thrombus), Peripheral nerve injury; Venous thrombosis (including deep vein thrombosis) and thromboembolism (including pulmonary embolism).