At Abbott, we are committed to advancing innovative technologies that improve the lives of people living with structural heart diseases. We strive to provide safe, effective, and minimally invasive treatment options that address the unique needs of these patients. We led the way in Transcatheter Edge-to-Edge Repair (TEER) with the development of the MitraClip™ and TriClip™ TEER Systems, offering transformative solutions for patients suffering from severe mitral regurgitation (MR) and symptomatic severe tricuspid regurgitation (TR). Building on the legacy of innovation, we reimagined the treatment of aortic stenosis with the Navitor™ TAVI** System. For patients with complex mitral valve disease and MR due to severe mitral annular calcification (MAC), the Tendyne System delivers a new path forward for patients with limited treatment choices.
Together, these innovations reflect Abbott’s leadership in structural heart therapies, empowering clinicians and transforming care for patients around the world.
**TAVI is also referred to as TAVR (Transcatheter Aortic Valve Replacement)
MitraClip™ TEER Therapy
MitraClip™ Transcatheter Edge-to-Edge Repair (TEER) procedure is a minimally invasive treatment option, uniquely designed for the mitral valve, for select patients with primary or secondary MR. MitraClip™ TEER clinical evidence set the foundation for TEER recommendations from ACC/AHA1, ESC/EACTS2-3 and ASPC4. As the standard of care in TMVr built on the only proven mitral valve therapy with over 20 years of clinical experience and more than 200,000 patients treated allowing physicians to treat more patients with more options.
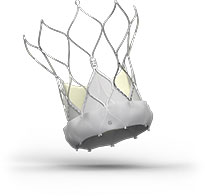
Navitor™ TAVI System
Advancing the forefront of innovative design, the Navitor™ Transcatheter Aortic Valve Implantation (TAVI) System brings together smart PVL-sealing technology, exceptional single-digit gradients,6 stable deployment, accurate valve placement and uncompromised coronary access to achieve excellent clinical outcomes in patients with severe aortic stenosis who are at high or greater risk for surgery.
Tendyne™ TMVR System
Tendyne™ Transcatheter Mitral Valve Replacement (TMVR) System is first-in-class technology designed to eliminate mitral regurgitation (MR), offering select patients with MR (≥ grade 3), a minimally invasive option for mitral valve replacement. The Tendyne TMVR system provides improvements in heart failure symptoms by consistently reducing MR.
TriClip™ TEER System
TriClip™ Transcatheter Edge-to-Edge Repair (TEER) is a minimally invasive treatment option for patients with symptomatic severe Tricuspid Regurgitation (TR). TriClip TEER offers exceptional safety7,8 and maximized effectiveness9,10 to significantly improve quality of life (QoL) and functional status of select patients with TR.11
References
- Heidenreich PA, et al. "2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association. Joint Committee on Clinical Practice Guidelines." J Am Coll Cardiol. Apr 01,2022. Epublished DOI: 10.1016/j.jacc.2021.12.012.
- Praz F, Borger M, Lanz J, et al., ESC/EACTS Scientific Document Group, 2025 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS), European Heart Journal, 2025, ehaf194, https://doi.org/10.1093/eurheartj/ehaf194
- McDonagh TA, Metra M, Adamo M, et al., ESC Scientific Document Group, 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC, European Heart Journal, Volume 42, Issue 36, 21 September 2021, Pages 3599–3726, https://doi.org/10.1093/eurheartj/ehab368
- Yeo KK, Tan JWC, Muller DW, et al. Asian Pacific Society of Cardiology Consensus Recommendations on the Use of MitraClip for Mitral Regurgitation. Eur Cardiol. 2021;16:e25. Published 2021 Jun 1. doi:10.15420/ecr.2021.01
- Data on File at Abbott.
- Smith, D. One-year clinical trial results with a next-generation aortic transcatheter heart valve. Presented at: EuroPCR conference; May 17-20, 2022.
- Tang GHL, Hahn RT, Whisenant BK, et al. Tricuspid transcatheter edge-to-edge repair for severe tricuspid regurgitation: 1-year outcomes from the TRILUMINATE randomized cohort. J Am Coll Cardiol. Published online 2025;85(3):235-246. doi:10.1016/j.jacc.2024.10.08.
- Lurz P, Rommel KP, Schmitz T, et al. Real-world 1-year results of tricuspid edge-to-edge repair from the bRIGHT study. J Am Coll Cardiol. 2024;84(7):607-616. doi:10.1016/j.jacc.2024.05.006.
- Kar S, Makkar RR, Whisenant BK, et al. Two-year outcomes of transcatheter edge-to-edge repair for severe tricuspid regurgitation: The TRILUMINATE Pivotal Randomized Trial. Circulation. March 30, 2025. doi:10.1161/CIRCULATIONAHA.125.074536.
- Estevez-Loureiro R. Real-world Outcomes for Tricuspid Edge-to-Edge Repair: Initial 2-year Outcomes from the bRIGHT Trial. Presented at PCR London Valves on November 19-21, 2023; London, UK.
- Nickenig G. Percutaneous edge-to-edge repair for tricuspid regurgitation: 3-year outcomes from the TRILUMINATE trial. Presented at PCR London Valves on November 19-21, 2023; London, UK.
MAT-2011954 v7.0