Clinical Evidence
The studies shown below are examples of the safety and benefits that Perclose ProGlide™ Suture Mediated Closure (SMC) may provide patients requiring small or large sheaths in arterial or venous access procedures.
Perclose Proglide™ SMCS vs. Manual Compression
A study showing ambulation times, costs and patient satisfaction between the two hemostasis approaches.
Study Overview
Femoral arterial closure using ProGlide™ is more efficacious and cost-effective when ambulating early following cardiac catheterization.
International Journal of Cardiology: Heart and Vasculature, 13 (2016) 6-13
Background
It is unclear whether early ambulation with Perclose ProGlide™ Suture-Mediated Closure System is safe or is associated with patient satisfaction and cost savings as compared with manual compression.
Objective
Evaluate the efficacy and cost-effectiveness of early ambulation (within 30 minutes) following femoral artery closure with the Perclose ProGlide™ SMCS in patients undergoing diagnostic cardiac catheterization compared with manual compression.
Method
Prospective, single-center study of 170 patients equally split into two arms measuring patient ambulation (20 feet within 30 minutes), time to hemostasis, and time to discharge. Using a fully allocated cost model, a cost analysis of both Perclose ProGlide™ SMCS and manual compression was performed. Also, a multivariate analysis was used to determine predictors of patient satisfaction.
Primary Endpoint
The primary endpoint was time-to-ambulation (TTA) following device closure.
Clinical Outcomes
| Perclose ProGlide™ SMCS (N = 85) | Manual Compression (N = 85) | P-Value | |
|---|---|---|---|
| Time to hemostasis (mean) | 1.5 min | 20.4 min | <0.001 |
| Time to ambulation (mean) | 27.1 min | 248.0 min | <0.001 |
| Time to discharge (mean) | 59.6 min | 349.9 min | <0.001 |
| Procedural success | 100% (85/85) | 96% (82/85) | 0.12 |
| Overall complications | 3.5% (3/85) | 11.8% (10/85) | 0.08 |
Cost Analysis
Perclose ProGlide™ SMCS provided nearly $1,000 in cost savings per patient compared to manual compression.
| Perclose ProGlide™ SMCS (N = 85) | Manual Compression (N = 85) | |
|---|---|---|
| Total procedural cost (without hemostasis device) | $564.5 ± 132.3 | $553.7 ± 121.0 |
| Hemostasis device cost1 | $278.2 ± 77.4 | $41.6 ± 22.4 |
| Post-procedural cath lab holding2 | $308.5 ± 78.8 | $1,190.8 ± 333.6 |
| Total adjusted nursing costs3 | $99.1 ± 41.1 | $389.5 |
| Hospital adjusted in-patient expenses4 | $0.0 | $2,052.6 ± 250.2 |
| Total cost | $1,250.3 ± 146.4 | $2,248.1 ± 910.2 |
| Incremental savings per patient | $983.6 | |
All data are U.S. dollars ($): mean ± SD.
- All patients in the device group (Perclose ProGlide™ SMCS) received a Perclose ProGlide™ SMCS device ($197.9/device) ± Neptune‡ Pads ($35.2/pad) while all in the manual compression group (Manual) received only Neptune‡ Pad(s).
- Based on an institutional post-procedural cath lab holding cost per hour of $205 per patient.
- Based on an adjusted nursing cost per hour of $67 per patient (extrapolated from adjusted annualized nursing salary per nurse per year).
- Based on hospital adjusted expenses per in-patient day of $1551
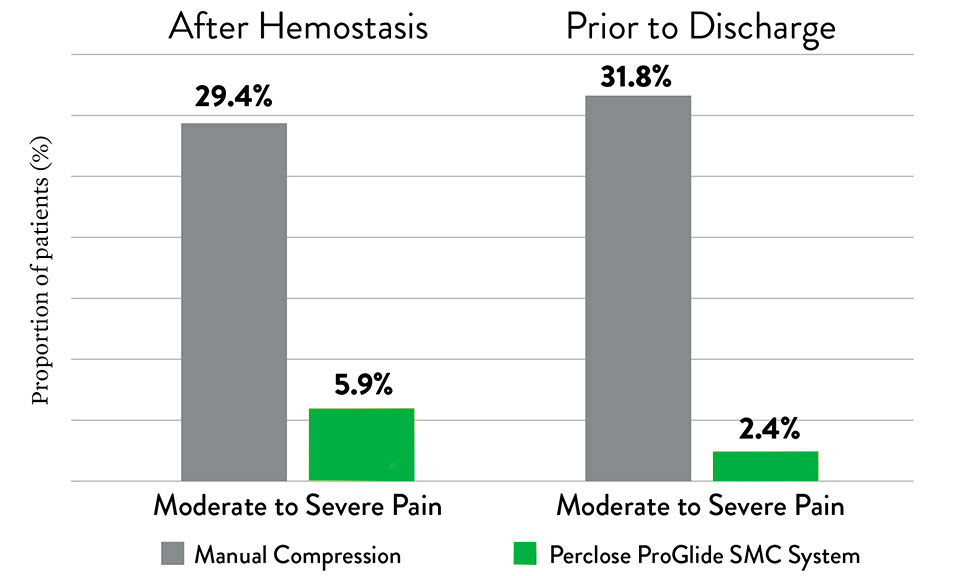
Patient Satisfaction
Perclose ProGlide™ SMCS helps reduce severe levels of patient discomfort as compared to manual compression.
Summary
- Perclose ProGlide™ SMCS provided significantly shorter times to hemostasis, ambulation, and discharge.
- Perclose ProGlide™ SMCS provided nearly $1,000 in cost savings per patient compared to manual compression.
- Perclose ProGlide™ SMCS helped reduce severe levels of patient discomfort as compared to manual compression.
Perclose ProGlide™ SMCS U.S. Large-Hole Study for EVAR
An overview of the Least Invasive Fast-Track EVAR (LIFE) Registry
Study Overview
Krajcer, Zvonimir. Fast-Track Endovascular Aortic: Final Results from the Prospective LIFE Registry.
VIVA 2016
Objective
Demonstrate the clinical and cost benefits associated with the ultra-low profile (14F) Ovation‡ Abdominal Stent Graft platform under the least invasive conditions, including percutaneous access, no general anesthesia, no ICU admission, and next-day discharge.
Method
Prospective, non-randomized, multi-center post-market study of 250 patients at 35 U.S. centers
Primary Endpoint
Major adverse event within 30 days (10.4% target performance goal)
Secondary Endpoint
- Treatment success (completion of Fast-Track protocol)
- Procedure, fluoroscopy, and anesthesia time; access complications; ambulatory status, hospital stay; quality of life
- Freedom from type I/III endoleak; conversion to open repair, rupture, AAA-related reintervention; mortality
Clinical Outcomes
Perclose ProGlide™ SMCS had a high technical success rate with no device or procedure-related major adverse events.
| Perclose ProGlide™ SMCS (n = 250) |  | |
| Technical success | 97.6% | |
| Device-related MAE | 0% | |
| Procedure-related MAE | 0% | |
| Hours to ambulation (median) | 7.9 Hours | |
| Hospital stay (mean) | 1.2 days |
MAE non-device non procedure-related: death due to acute respiratory failure 28 days post procedure
Cost Analysis
The Fast-Track EVAR approach demonstrates $21,100 in savings as compared to the Standard EVAR approach, with the hospital stay contributing to the majority of the amount.
| Standard EVAR1,2 | Fast-Track EVAR | Fast-Track Savings | |
|---|---|---|---|
| Anesthesia | General, 84% $500 | Local, 100% $300 | $200 |
| Access | Cutdown3 $300 | Bilateral PEVAR $1,200 | ($900) |
| ICU | 1.4 Days, 51% $15,300 | 0 Days, 0% $0 | $15,300 |
| Non-ICU | 2.3 Days $12,900 | 1.2 Days $6,700 | $6,200 |
| 30-Day Reintervention | $29,400, 1.1% $300 | 0% $0 | $300 |
| Total | $29,300 | $8,200 | $21,100 |
- Standard EVAR Control group: Benchmarked 8,036 patients treated with standard, elective infrarenal EVAR at an alliance of ~3,750 U.S. Premier hospital facilities, based on Inpatient Discharge between 2012-2015
- Average costs per patient, extracted costs for access, anesthesia, ICU and hospital stay to calculate costs associated with Fast-Track
- 30% applicability based on anatomic criteria, with 23% bilateral / 7% unilateral PEVAR (Manunga et al, J Vasc Surg, 2013)
Summary
Perclose ProGlide™ SMCS had a high technical success rate with no device- or procedure-related major adverse events.
The Fast-Track EVAR approach demonstrates $21,100 in savings as compared to the Standard EVAR approach, with the longer hospital stay contributing to the majority of the difference.
MAT-2202615 v2.0
Perclose ProGlide™ Suture-Mediated Closure (SMC) System

Indications:
The Perclose ProGlide™ Suture-Mediated Closure System is indicated for the percutaneous delivery of suture for closing the common femoral artery and vein access site of patients who have undergone diagnostic or interventional catheterization procedures.
The Perclose ProGlide™ SMC System is indicated for closing the common femoral vein in single or multiple access sites per limb.
The Perclose ProGlide™ SMC System is used without or, if required, with adjunctive manual compression.
For access sites in the common femoral artery using 5F to 21F sheaths. For arterial sheath sizes greater than 8F, at least two devices and the pre-close technique are required.
For access sites in the common femoral vein using 5F to 24F sheaths. For venous sheath sizes greater than 14F, at least two devices and the pre-close technique are required.
Caution:
Federal law restricts this medical device to sale by or on the order of a physician (or allied healthcare professionals, authorized by, or under the direction of, such physicians) who is trained in diagnostic and / or interventional catheterization procedures and who has been trained by an authorized representative of Abbott.
Prior to use, the operator must review the Instructions for Use and be familiar with the deployment techniques associated with the use of this device.
During closure of access sites using a procedural sheath greater than 8F, it is recommended that a vascular surgeon or a surgeon with vascular training be available in case surgical conversion to control bleeding and to repair the vessel is needed.
Contraindications:
There are no known contraindications to the use of this device.
Warnings:
Do not use the Perclose ProGlide™ SMC System if the packaging or sterile barrier has been previously opened or damaged or if the components appear to be damaged or defective.
DO NOT RESTERILIZE OR REUSE. The Perclose ProGlide™ SMC System is intended for single use only.
Do not use the Perclose ProGlide™ SMC System if the sterile field has been broken where bacterial contamination of the sheath or surrounding tissues may have occurred, since such a broken sterile field may result in infection.
Do not use the Perclose ProGlide™ SMC System if the puncture site is located above the most inferior border of the inferior epigastric artery (IEA) and / or above the inguinal ligament based upon bony landmarks, since such a puncture site may result in a retroperitoneal hematoma. Perform a femoral angiogram to verify the location of the puncture site. Note: This may require both a right anterior oblique (RAO) and left anterior oblique (LAO) angiogram to adequately visualize where the sheath enters the femoral vessel.
Do not use the Perclose ProGlide™ SMC System in arterial or venous access if the puncture is through the posterior wall or if there are multiple punctures in the same access site, since such punctures may result in a hematoma or retroperitoneal bleed.
Do not use the Perclose ProGlide™ SMC System if the puncture site is located in the superficial femoral artery or the profunda femoris artery, or the bifurcation of these vessels, since such puncture sites may result in a pseudoaneurysm, intimal dissection, or an acute vessel closure (thrombosis of small artery lumen). Perform a femoral angiogram to verify the location of the puncture site. Note: This may require both a right anterior oblique (RAO) and left anterior oblique (LAO) angiogram to adequately visualize where the sheath enters the femoral vessel.
Precautions:
- Prior to use, inspect the Perclose ProGlide™ SMC System to ensure that the sterile packaging has not been damaged during shipment. Examine all components prior to use to verify proper function. Exercise care during device handling to reduce the possibility of accidental device breakage.
- As with all catheter-based procedures, infection is a possibility. Observe sterile technique at all times when using the Perclose ProGlide™ SMC System. Employ appropriate groin management, as per hospital protocol, post procedure and post hospital discharge to prevent infection.
- Use a single wall puncture technique. Do not puncture the posterior wall of the vessel in arterial and venous access.
- Do not deploy the Perclose ProGlide™ Device at an elevated angle against resistance as this may cause a cuff miss or device breakage.
- There are no reaccess restrictions if previous arteriotomy / venotomy repairs were achieved with Abbott Medical SMC or SMCR systems.
- If significant blood flow is present around the Perclose ProGlide™ Device, do not deploy needles. Remove the device over a 0.038" (0.97 mm) (or smaller) guide wire and insert an appropriately sized sheath.
- Prior to depressing the plunger to advance the needles, stabilize the device by the body to ensure the foot is apposed to the vessel wall and the device does not twist during deployment. Twisting (torquing) of the device could lead to needle deflection resulting in a cuff miss. Do not use excessive force or repeatedly depress the plunger. Excessive force on the plunger during deployment could potentially cause breakage of the device, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- Do not apply excessive force to the lever when opening the foot and returning the foot to its original position down to the body of the device. Do not attempt to remove the device without closing the lever. Excessive force on the lever or attempting to remove the device without closing the lever could cause breakage of the device and / or lead to vessel trauma, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- Do not advance or withdraw the Perclose ProGlide™ Device against resistance until the cause of that resistance has been determined. Excessive force used to advance or torque the Perclose ProGlide™ Device should be avoided, as this may lead to significant vessel damage and / or breakage of the device, which may necessitate intervention and / or surgical removal of the device and vessel repair.
- If excessive resistance in advancing the Perclose ProGlide™ Device is encountered, withdraw the device over a 0.038" (0.97 mm) (or smaller) guide wire and reinsert the introducer sheath or use manual compression.
- Remove the Perclose ProGlide™ sheath before tightening the suture. Failure to remove the sheath prior to tightening the suture may result in detachment of the tip of the sheath.
- Care should be taken to avoid damage to the suture from handling. Avoid crushing damage due to application of surgical instruments such as clamps, forceps or needle holders.
- For catheterization procedures using a 5F – 8F procedural sheath, use manual compression in the event that bleeding from the femoral access site persists after the use of the Perclose ProGlide™ SMC System to obtain hemostasis.
- For catheterization procedures using a procedural sheath > 8F, use manual compression, compression assisted devices, surgical repair, and / or other appropriate treatment methods in the event that bleeding from the femoral access site persists after the use of the Perclose ProGlide™ SMC System to obtain hemostasis.
- For catheterization procedures using a procedural sheath > 8F, where the operating physician is not a vascular surgeon, it is recommended that a vascular surgeon or a surgeon with vascular training be available during the procedure to perform any necessary vascular surgical intervention.
- If the Perclose ProGlide™ Device is used to close and repair multiple access sites in the same vessel, space the access sites apart adequately to minimize sheath-device interference
Potential Adverse Events:
Potential adverse events associated with use of vessel closure devices may include, but are not limited to, the following:
- Allergic reaction or hypersensitivity to device components
- Vascular access complications which may require transfusion or vessel repair, including:
- Anemia
- Aneurysm
- Arteriovenous fistula
- Bleeding / hemorrhage / re-bleeding
- Bruising
- Hematoma
- Embolism
- Inflammation
- Intimal tear / dissection
- Perforation
- Pseudoaneurysm
- Retroperitoneal hematoma / bleeding
- Scar formation
- Wound dehiscence
- Cardiac arrhythmias (including conduction disorders, atrial and ventricular arrhythmias)
- Atrial arrhythmias
- Ventricular arrhythmias
- Femoral artery / venous complications which may require additional intervention, including:
- Arterial / venous stenosis
- Arterial / venous occlusion
- Arteriovenous fistula
- Intimal tear / dissection
- Ischemia distal to closure site
- Nerve injury
- Numbness
- Thrombus formation
- Vascular injury
- Venous thromboembolism (including deep vein thrombosis, pulmonary embolism, post-procedure pulmonary embolism)
- Infection – local or systemic
- Pain
- Hemodynamic instability:
- Hypotension / hypertension
- Vasovagal episode
- Death
- Device complications
- Device failure
- Device malfunction
MAT-2100358 v4.0
