Clinical Evidence
Discover how the Perclose ProGlide™ System is clinically effective across a variety of procedures and specialties:
Clinical Trials
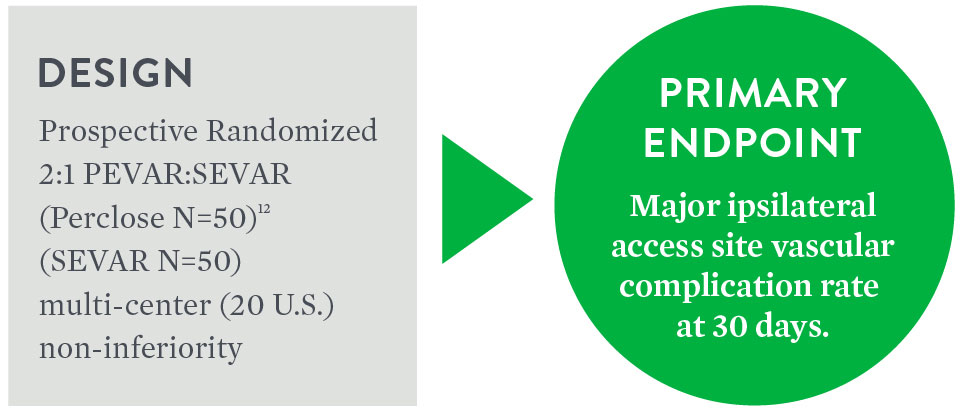
PEVAR Clinical Trial
Objective: To compare the safety and effectiveness of the 'pre-close' technique for percutaneous femoral artery (FA) access and closure (PEVAR) to Surgical Cutdown (SEVAR).
Key Findings
- Mean procedure time was reduced in PEVAR patients by 34 minutes (106.5 ± 44.9 minutes PEVAR vs. 141.1 ± 73.4 minutes SEVAR, p=.0056)1
- Mean time to hemostasis was reduced following PEVAR by 13 minutes (9.8 ± 17.0 minutes PEVAR vs. 22.7 ± 22.9 minutes SEVAR, p=.0023)1
Primary Endpoint Achieved
Major6 Ipsilateral Vascular Access
Complications at 30 Days
| PEVAR Perclose N=50 | SEVAR N=50 | Difference 95% CI3 | p-value3 | |
|---|---|---|---|---|
| Major Complications [95% CI]7 | 6% (3/50) [1.3%, 16.5%] | 10% (5/50) [3.3%, 21.8%] | -4.0% [-, 4.9%] | 0.0048 |
Composite of Minor8 Ipsilateral Vascular Access
Complications at 30 Days
| PEVAR Perclose N=50 | SEVAR N=50 | p-value3 | |
|---|---|---|---|
| Minor Ipsilateral Access Site Vascular Complications at 30 Days [95% CI]7 | 4% (2/50) [0.5%, 13.7%] | 8% (4/50) [2.2%, 19.2%] | 0.67774 |
Source: Nelson, Peter R. et al. A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). Journal of Vascular Surgery. (2014): 1181-1193.
- By two-sample t-test.
- PEVAR-Perclose analyzed independently from Prostar™ XL.
- One-sided p-value and 95% confidence interval for non-inferiority test by using asymptotic test statistics with non-inferiority margin of 10%.
- No statistically significant difference.
- By Fisher's exact test.
- Major Complications: access site vascular injury requiring repair; new onset lower extremity ischemia requiring surgical or percutaneous intervention; Access site-related bleeding requiring transfusion; access site-related infection requiring IV antibiotic or prolonged hospitalization; access site-related nerve injury that is permanent or requires surgery.
- By Clopper-Pearson exact confidence interval.
- Minor Complications: pseudoaneurysm or AV fistula; hematoma ≥ 6 cm; post-discharge bleeding requiring > 30 minutes to re-achieve hemostasis; lower extremity arterial emboli or stenosis attributed to access site; deep vein thrombosis; access site-related vascular laceration; transient access site-related nerve injury; access site wound dehiscence; access site-related lymphocele; localized access site infection treated with IM or PO antibiotic.
Clinical Evidence on Closure of Large-Bore Venous Access
Perclose™ Device Cohort in the REALISM9 Clinical Trial
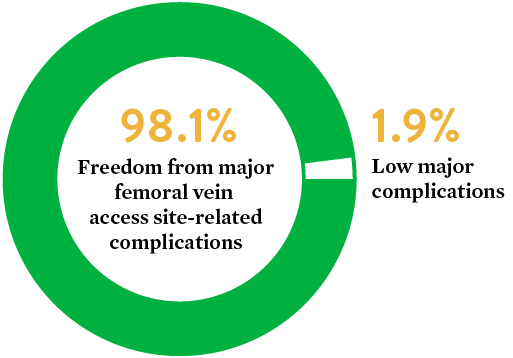
A prospective analysis was performed to evaluate the safety and effectiveness of Perclose™ Devices in closing large-sized venous access sites through a retrospective data collection. The prospective analysis included subjects in whom Perclose™ Devices were used as the primary method for large-bore venous access site closure during the TMVr index procedure with a 24F vascular sheath.
Key Findings
- Major complication was low at 1.9%
- Freedom from major femoral vein access site-related complications was 98.1% at 30 days
- Perclose™ Devices are safe and effective in the closure of venous access-site with up to 24F sheath
Source: The Use of the Perclose ProGlide™ Suture Mediated Closure (SMC) Device for Venous Access-Site Closure up to 24F Sheaths. Kar, Saibal; Hermiller, James; et al. CRT 2018.
9. EVEREST II/REALISM Continued Access Registry Study.
Real-World Evidence on Repair of Large-Bore Arterial Access
Perclose™ Device vs. Surgical Cutdown
The Perclose™ Device vs. Surgical Cutdown retrospective study is designed to compare clinical outcomes and complication rates among patients undergoing closure of large-bore arterial access using Perclose™ Devices versus Surgical Cutdown (Cutdown) in a real-world setting.
Key Findings
The use of Perclose™ Devices for repair of large-bore arterial access is associated with significantly lower blood transfusions, infections, mortality, and length of stay compared to Surgical Cutdown.
Patient Baseline
| Cutdown | Perclose | |
|---|---|---|
| # of Patients | 757 | 757 |
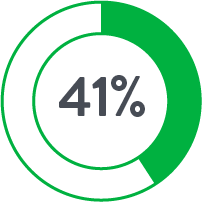
| Anticoagulants◊ | 17.8% | 44.9% |
◊p<0.05
Perclose™ Device Patients
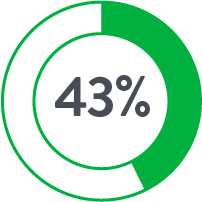
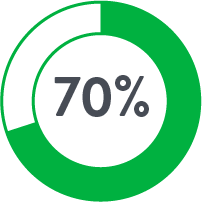
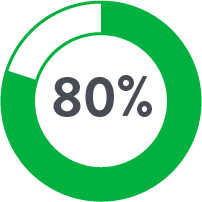
Source: Perclose ProGlide™ Versus Surgical Closure Outcomes – Real World Evidence. Schneider, Darren B; Krajcer, Zvonimir; et al. LINC 2018.
PEVAR Publications
Italian Percutaneous EVAR (IPER) Registry
Outcomes of 2,381 percutaneous femoral access sites’ closure for aortic stent-graft
Objective: The aim of this publication was to report outcomes of endovascular aneurysm repair with percutaneous femoral access (PEVAR) using Prostar™ XL and Perclose™ Devices closure systems, from the multi-center Italian Percutaneous EVAR (IPER) registry.
Method
- Prospective
– 7 centers
– Operators with at least 50 closure device deployments - January 2010 – December 2014
– Use of Perclose began in 2013 - 2,381 femoral access sites
– 514 Perclose closes
– 1,867 Prostar closes - > 18F sheath size: 26.5%
Perclose™ Devices-Only Result
- 97.5% (501/514) technical success rate10
Total Results including Prostar
- 3.2% (76/2,381) major complications11 requiring surgical cutdown
- 1.1% (27/2,381) minor complication12
- 0.25% (6/2,381) puncture site complication observed during one-month follow-up
Key Takeaways
- High device success (Perclose™ Devices 97.5%) even with demanding cases due to the presence of obesity (24.7%), femoral artery calcification (53%), and iliac tortuosity (22.6%)
- First and largest prospective study carried out on a cohort of unselected patients undergoing PEVAR with different endografts in well-trained centers by highly experienced operators
- Femoral calcification was the only independent predictor of technical failure
Source: Pratesi, G., et al. "Italian Percutaneous EVAR (IPER) Registry: outcomes of 2,381 percutaneous femoral access sites' closure for aortic stent-graft." The Journal of Cardiovascular Surgery 56.6 (2015): 889-898.
10. Technical success was defined as the ability to obtain a successful percutaneous vascular access and closure without serious complications.
11. Major access-related complications included all the complications requiring an early surgical conversion due to bleeding, pseudoaneurysm, CFA stenosis/occlusion, and were considered a technical failure of system closure.
12. Minor access-related complications were related to the presence of minimal bleeding at the access site not requiring surgical conversion.
Perclose™ Devices in PEVAR and TAVI – Brazil Study
Objective: Assessment of efficacy, complication, and potential risk factors for Perclose™ Devices
Method
- 123 Patients
– 47.6% TAVI – 33.5% EVAR
– 13.7% TEVAR, 3.2% TAAA - 242 vascular access sites
- Single-center retrospective study
- ≥ 18F sheath size: 54.3%
- Single operator with 50 small-hole closure experience
(no prior large-hole experience) - Angiotomography instead of ultrasound used for puncture
Perclose™ Device Results
- 98.37% (121/123) patient technical success rate13
- 1.62% acute major complication rate
– Failures had > 2/3 CFA calcification - 0.81% (1/123) minor complication observed at 30-day follow-up
- Operator encountered an initial learning curve with the 3 complications occurring during the first 35 cases
Key Takeaways
- Experience using Perclose™ Devices in small-hole closure help with large-hole closure success
- Complications occur more often in unfavorable access site anatomy with proper patient selections (facilitated by preoperative imaging) improving success rates.
Source: Saadi, Eduardo Keller, et al. "Totally Percutaneous Access Using Perclose ProGlide™ for Endovascular Treatment of Aortic Diseases." Brazilian Journal of Cardiovascular Surgery 32.1 (2017): 43-48.
13. Device failure defined as failure to achieve hemostasis at the access site, leading to alternative treatment other than manual compression.
Perclose™ Devices in PEVAR – Taiwan Study
Objective: Evaluate the outcomes and predictive factors for additional Perclose™ Device deployments
Method
- 268 patients, 418 vascular access sites
- 9 operators
– 3 were within the learning curve for Perclose14 - Factors measured
– Depth
– Sheath size
– CFA diameter
– Any CFA calcification- Anterior wall
- Lateral wall
- Medial wall
- Posterior wall
Perclose™ Device Results
- 87.6% (366/418) Primary technical success15 with two Perclose
- 99% secondary technical success16 with additional Perclose device
– 38 required one additional device and 10 required two additional devices - Only anterior CFA wall calcification was a significant predictor for additional Perclose deployment
Key Takeaways
- It is important to perform a femoral angiogram to evaluate the severity and location of calcification at the puncture site
- Even with additional device deployment, high success rate was able to be achieved even in the presence of anterior wall calcification (17% of total access sites)
Source: Lin, Shen-Yen, et al. "Predictive Factors for Additional Perclose Deployment in Percutaneous Endovascular Aortic Repair." Journal of Vascular and Interventional Radiology 28.4 (2017): 570-575.
14. Learning curve shown to be most pronounced over first 18 months. Bechara C.F. et al.: Predicting the learning curve and failures of total percutaneous endovascular aortic aneurysm repair. J Vasc Surg 2013; 57: pp. 72-76.
15. Primary technical success rate defined as the ability to achieve hemostasis without additional ProGlide™ device deployment or surgical repair.
16. Secondary technical success rate defined as the ability to achieve hemostasis after deployment of one or two ProGlide™ devices without surgical repair.
Perclose™ Devices in PEVAR Trial
Objective: To evaluate the safety and effectiveness of the “pre-close” technique for PEVAR compared to surgical cutdown (SEVAR)
Method
- Prospective, randomized, 20 U.S. centers
- Perclose N=50, SEVAR N=50, Prostar N=51
- Primary endpoint: Procedural technical success17 and major complications at 30 days
- Noninferiority trial design
Result
| Perclose PEVAR | SEVAR | p-value | |
|---|---|---|---|
| Major Complications | 6% (3/50) | 10% (5/50) | 0.0048 |
| Procedure Time | 106.5 ± 44.9 min | 141.1 ± 73.4 | 0.0056 |
| Time to Hemostasis | 9.8 ± 17.0 | 22.7 ± 22.9 | 0.00233 |
Key Takeaways
- It is important to perform a femoral angiogram to evaluate the severity and location of calcification at the puncture site
- Even with additional device deployment, high success rate was able to be achieved even in the presence of anterior wall calcification (17% of total access sites)
Source: Nelson, Peter R., et al. "A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial)." Journal of Vascular Surgery 59.5 (2014): 1181-1193.
17. Procedural technical success defined as successful vascular access and closure per randomized assignment, and successful endograft delivery, deployment, and catheter removal, without serious complication or need for vascular exposure in the percutaneous group.
Perclose™ Devices LIFE Registry
Objective: Demonstrate clinical and cost benefits when utilizing a Fast-Track EVAR protocol with the Ovation stent graft system
Method
- Prospective multi-center registry
- 250 patients, 35 U.S. centers
- 14F Ovation Abdominal Stent Graft
- Fast-Track EVAR Protocol
– Percutaneous access
– No general anesthesia
– No ICU admission
– Next-day discharge
Perclose™ Device Results
- 97.6% Perclose technical success18 rate (244/250)
- No device-related or procedure-related major adverse events
- Cost analysis shows Fast-Track EVAR is less costly than standard EVAR
Cost Analysis
| Fast Track | Standard EVAR*** | Fast-Track Savings | |
|---|---|---|---|
| Anesthesia | Local/Regional $300 | General $500 | $200 |
| Access | Bilateral PEVAR $1,200 | Cutdown† $300 | ($900) |
| ICU | 0% $0 | 1.4 Days, 51% $15,300 | $15,300 |
| Non-ICU | 1.2 Days $6,700 | 2.3 Days $12,900 | $6,200 |
| 30D Reintervention | 0% $0 | $29.4K, 1.1% $300 | $300 |
| Total | $8,200 | $29,300 | $21,100 |
**Standard EVAR Control group: Benchmarked 8,306 patients treated with standard, elective infrarenal EVAR at an alliance of ~3,750 U.S. Premier hospital facilities, based on Inpatient Discharge between 2012-2015.
***Standard EVAR: Average costs per patient, extracted costs for access, anesthesia, ICU, and hospital stay to calculate costs associated with Fast-Track.
†Assumes 30% applicability based on anatomic criteria, with 23% bilateral PEVAR and 7.0% unilateral PEVAR (Manunga et al., J Vasc Surg. 2013).
Key Takeaways
- Fast-Track is less costly than standard EVAR with hospital and ICU stay as the main cost drivers
- Patient selection is critical to success as femoral arteries should be free of heavy calcification or extreme tortuosity to facilitate bilateral percutaneous access
Source: Kracjcer, Z. Fast-Track Endovascular Aortic Repair: Final Results from the Prospective LIFE registry. VIVA 2016.
18. Technical Success (Successful Bilateral PEVAR)
TAVI Publications
Perclose™ Devices in TAVI – CONTROL Study
Objective: To evaluate the efficacy of Perclose™ Devices
Method
- Retrospective study
- 9 centers in Europe, North America, and the Middle East
- 472 Perclose compared to 472 Prostar deployment
- TAVI devices used: Sapien (51.1%) and CoreValve (43.2%)
- 18.3 ± 1.7F mean sheath size
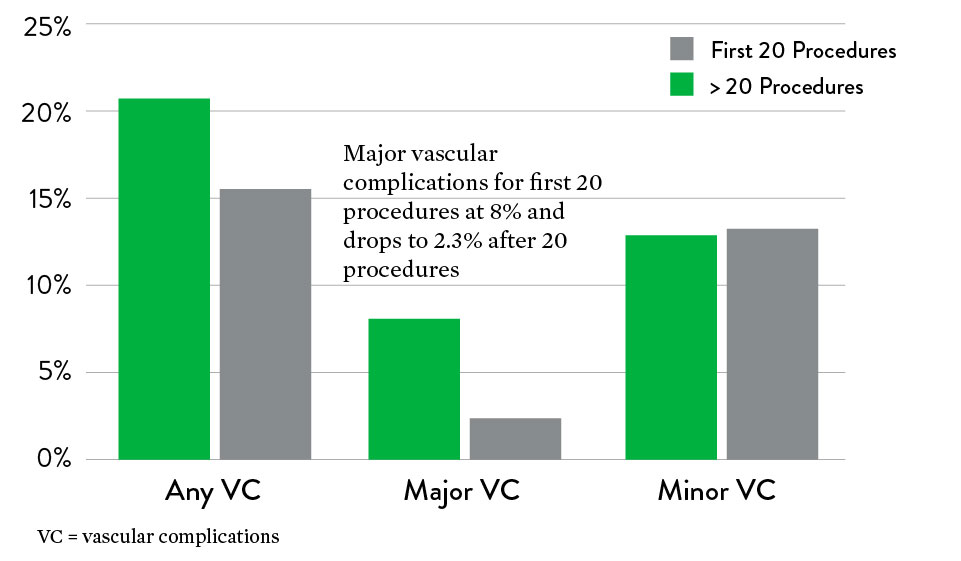
Perclose™ Device-Only Result
- 1.9% (9/472) major complications
- 18% (85/472) minor complications
- There was a significant decrease in major vascular complications after the first 20 cases, showing a learning curve effect
Key Takeaways
- Low major vascular complication rate in TAVI procedures – 1.9%
- Major complication rate decreased significantly after first 20 procedures, showing a learning curve effect
Source: Barbash, Israel M., et al. "Comparison of vascular closure devices for access site closure after transfemoral aortic valve implantation." European Heart Journal 36.47 (2015): 3370-3379.
Perclose™ Devices in TAVI – Germany Study
Objective: Evaluate the safety and efficacy of Perclose™ Devices
Method
- Prospective single-center study
- 348 Perclose patients compared to 237 Prostar patients
- TAVI devices used
– Sapien 3
– Lotus
– CoreValve - Sheath size 23.7F ± 1.5F
Perclose™ Device-Only Result
- 95.4% (332/348) technical success rate19
- 2.3% (8/348) major vascular complications
- 5.7% (20/472) minor vascular complications
Key Takeaways
- High technical success rate
- Low major vascular complication in TAVI procedures
Source: Seeger, Julia, et al. “Impact of suture mediated femoral access site closure with the Prostar XL compared to the Perclose system on outcome in transfemoral aortic valve implantation.” International Journal of Cardiology 223 (2016): 564-567.
19. Device failure defined as failure to achieve hemostasis at the access site leading to alternative treatment other than manual compression. 4.6% (n=16) needed stenting for device failure.
Perclose™ Devices in PEVAR and TAVI – Brazil Study
Objective: Assessment of efficacy, complication, and potential risk factors for Perclose™ Devices
Method
- 123 Patients
– 47.6% TAVI
– 33.5% EVAR
– 13.7% TEVAR, 3.2% TAAA - 242 vascular access sites
- Single-center retrospective study
- ≥ 18F sheath size: 54.3%
- Single operator with 50 small-hole closure experience (no prior large-hole experience)
- Angiotomography instead of ultrasound used for puncture
Perclose™ Device-Only Result
- 98.37% (121/123) patient technical success rate20
- 1.62% acute major complication rate
– Failures had > 2/3 CFA calcification - 0.81% (1/123) minor complication observed at 30-day follow-up
- Operator encountered an initial learning curve with the 3 complications occurring during the first 35 cases
Key Takeaways
- Experience using Perclose™ Devices in small-hole closure help with large-hole closure success
- Pre-operative imaging on the puncture site to assess calcification important for device success
Source: Saadi, Eduardo Keller, et al. "Totally Percutaneous Access Using Perclose ProGlide™ for Endovascular Treatment of Aortic Diseases." Brazilian Journal of Cardiovascular Surgery 32.1 (2017): 43-48.
20. Device failure defined as failure to achieve hemostasis at the access site, leading to alternative treatment other than manual compression.
TMVr Publications
Prospective Analysis for Large-Bore Venous Access
The Use of the Perclose ProGlide™ Suture-Mediated Closure (SMC) Device for Venous Access Site
Safety and performance of Perclose ProGlide™ vascular closure device in managing large-hole venous access site
Objective: The primary objective of this study was to evaluate the safety and performance of ProGlide™ in the closure of the venous access site in subjects treated with a large-caliber femoral vein sheath (24F).
MAT-2414807 v1.0
