Objective Decisions.
Simplified Workflow.
PressureWire™ X Guidewire offers objective decisions with a simplified workflow.1
The innovative PressureWire™ X Guidewire—the world’s only wireless physiology wire2—can measure pressure and temperature to calculate Resting Full-Cycle Ratio (RFR), Fractional Flow Reserve (FFR), Index of Microvascular Resistance (IMR), and Coronary Flow Reserve (CFR). The guidewire’s fully integrated, secure, wireless measurements are integral to a cardiac cath lab’s clinical physiology routine. The PressureWire™ X Guidewire and CoroFlow‡ Cardiovascular System is the only full physiology solution in the cath lab, able to detect both epicardial disease and Coronary Microvascular Dysfunction (CMD).1-4



Objective Decisions
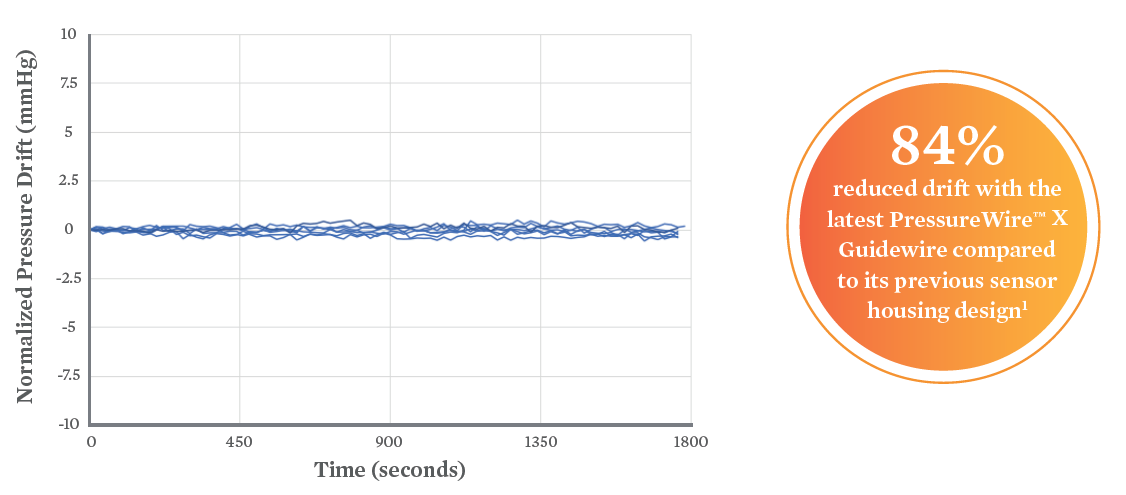
- Innovative sensor housing design offers <1 mmHg/30 min with reduction in drift due to microbubbles5,6
- Objective decision-making with both RFR and FFR options
- RFR has been studied in over 3,500 lesions and 2,000 patients7-12 to show diagnostic equivalence to iFR‡
Simplified Workflow
- The PressureWire™ X Guidewire is the world’s only wireless physiology wire2
- Wireless: from zero to go in 2 easy steps2
- Ideal for the modern radial approach
- Compatible with the Ultiri™ Measurement System with an easy touchscreen workflow
- Compatible with OPTIS™ Next Imaging Systems for imaging and physiology needs on one platform
- Compatible with the CoroFlow‡ Cardiovascular System for full physiology (assessment of both epicardial disease and microvascular dysfunction)12

2 Steps to Connect the Wireless
PressureWire™ X Guidewire1
- Zero. Zero the PressureWire™ X Guidewire by turning on the transmitter.
- Go. The transmitter’s function indicator shall display a stable green light when it is successfully zeroed.
References
- Data on file at Abbott.
- Volcano Corp. Verrata‡ guidewire Instructions for Use (IFU) and PrimeWire Prestige‡ Plus guidewire IFU. Opsens Inc. OptoWire‡ guidewire IFU and OptoWire‡ II guidewire IFU. ACIST Medical Systems. Navvus‡ Microcatheter IFU. Boston Scientific Corporation. Comet‡ guidewire IFU. PressureWire™ X Guidewire IFU. Refer to IFUs for additional information.
- Ford TJ, Stanley B, Sidik N, et al. 1-year outcomes of angina management guided by invasive coronary function testing (CorMicA). JACC Intv. 2020; 13:33-45.
- Pijls NH, Fearon WF, Tonino PA, et al. Fractional Flow Reserve Versus Angiography for Guiding Percutaneous Coronary Intervention in Patients with Multivessel Coronary Artery Disease. 2-Year Follow-Up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) Study. JACC. 2010; 56(3): 177-184.
- Data on file at Abbott.
- Kern et al. Comparing FFR Tools: New Wires and a Pressure Microcatheter. Cath Lab Digest. May 2016
- Jeremias A, et al. Resting full-cycle ratio (RFR): a novel physiologic index compared to Fractional Flow Reserve (FFR) in assessing the hemodynamic severity of a coronary stenosis: ILUMIEN I + PREDICT. EuroPCR 2018.
- Svanerud J, et al. Validation of a novel non-hyperemia index of coronary artery stenosis severity: the Resting Full-cycle Ratio (VALIDATE RFR) study. EuroIntervention. 2018;14:806-814.
- Ahn JM, et al. IRIS FFR: prognostic performance of five resting pressure-derived indexes of coronary physiology. TCT 2018.
- Kumar G. et al. Re-Validate Study. CRT 2019.
- Lee J-M, et al. Physiological and clinical assessment of resting physiological indexes. Circulation. 2019;139.
- CoroFlow‡ Cardiovascular System Instructions for Use (IFU). Refer to IFU for additional information.
MAT-2313414 v1.0
PressureWire™ X Guidewire

Indications: The PressureWire™ X Guidewire is indicated to direct a catheter through a blood vessel and to measure physiological parameters in the heart and in the coronary and peripheral blood vessels. Physiological parameters include blood pressure. The PressureWire™ X Guidewire can also measure blood temperature.
Contraindications: This guidewire is contraindicated for use in the cerebral vasculature.
Warnings:
- No modification of this device is allowed.
- The PressureWire™ X Guidewire is supplied sterile. Discard the guidewire if the pouch is opened or damaged, compromising the sterile barrier. The guidewire is designed for single use only and shall not be reused or resterilized. Adverse effects of using a non-sterile or resterilized guidewire may include, but are not limited to:
- Local and/or systemic infection
- Mechanical damage
- Inaccurate readings
- Observe all guidewire movements. Whenever the guidewire is moved or torqued, the tip movement should be examined under fluoroscopy. Never push, withdraw, or torque the guidewire if it meets resistance or without observing corresponding movement of the tip, otherwise vessel/ventricle trauma may occur.
- Torquing or excessive manipulation of the guidewire in a sharp bend, against resistance, or repeated attempts to cross a total vessel occlusion may:
- Cause dissection or perforation of blood vessels
- Cause vessel spasm
- Damage and/or fracture the guidewire
- When introducing the guidewire, flush the catheter and administer anticoagulation as for a standard catheterization procedure or clotting may occur.
- Do not use the guidewire in the ventricles if the patient has a prosthetic mechanical or biological valve. It may result in damage to both the prosthesis and the guidewire, which may cause injury or death.
- Use of the PressureWire™ X Guidewire in conjunction with interventional devices with a short rapid exchange may result in a folded or fractured guidewire.
- High frequency surgical devices must not be used on a patient at the same time as the guidewire.
Precautions:
- The PressureWire™ X Guidewire is a delicate instrument and should be handled carefully.
- Make sure that the transmitter is kept dry to ensure accurate pressure and/or temperature readings. Inaccurate readings may necessitate device replacement.
- Do not use the guidewire in conjunction with atherectomy catheters. It may damage the guidewire.
- Do not withdraw or manipulate the guidewire in a sharp-edged object. It may result in abrasion of the guidewire coating.
- Factors that may affect the accuracy of the diagnostic information include, but are not limited to:
- Improper placement of the aortic pressure sensor.
- Failure to achieve maximum coronary and myocardial hyperemia in FFR procedures.
- Blood flow affected by the position of interventional devices, such as balloon catheters.
- Guidewire readings may be affected by defibrillation. Rezero the guidewire after defibrillation use.
- Do not measure pressure when the guidewire sensor element is in a sharp bend or in contact with atrial or ventricular walls. It might result in pressure artifacts.
- Do not use the PressureWire™ X Guidewire together with another guidewire, for so called jailed wire technique, due to difficulty in guidewire withdrawal and possible guidewire entrapment.
- Store at room temperature (15°C – 25°C) in a dry and dark place.
Potential Adverse Events: Potential complications which may be encountered during all catheterization procedures include, but are not limited to: vessel dissection or occlusion, perforation, embolus, spasm, local and/or systemic infection, pneumothorax, congestive heart failure, myocardial infarction, hypotension, chest pain, renal insufficiency, serious arrhythmias, or death.
In addition, this device has a coating containing Polyethylene Glycol (PEG); potential allergic reactions (anaphylaxis) may occur during the interventional procedure if the patient is allergic to PEG.
MAT-2103599 v2.0
Coroventis‡ CoroFlow‡ Cardiovascular System

Indications: CoroFlow‡ is indicated to provide hemodynamic information for use in the diagnosis of patients with cardiovascular diseases.
CoroFlow‡ is intended for use in catheterization and related cardiovascular specialty laboratories to compute and display various physiological parameters based on the output from one or more measuring devices.
Contraindications: The system has no patient alarm functions. Do not use for cardiac/vital signs monitoring.
Warnings:
- If CoroFlow‡ is used together with 3rd party infusion catheters for assessment of Absolute Flow and Resistance, ensure that the maximum infusion rate per manufacturers instruction is not exceeded or vessel injury may occur.
- Do not use the CoroFlow‡ Cardiovascular System if there is reason to believe the system's security has been compromised or if the system was unaccounted for a period of time (i.e. misappropriated, modified or tampered with).
- Do not leave the CoroFlow‡ Cardiovascular System unattended when logged in as a PC Administrator.
- To protect the privacy and security of sensitive information, including electronic protected health information (EPHI), and to protect the integrity of the system itself, the system should be located in a physically secure, access-controlled environment.
- To protect the privacy and security of sensitive information, including electronic protected health information (EPHI), the PC on to which CoroFlow‡ is installed must be configured according to the Installation Instructions in this manual. Failure to configure the PC correctly may result in increased risk for unauthorized release of protected health information. Windows settings include:
- Activation and configuration of restricted user Access
- Activation of Windows Firewall and blocking of network connections
- Activation of Windows Bitlocker drive encryption
- Activation of Windows Secure Boot
- Activation of Windows Anti-Virus scanning and ransomware protection. Ensure CoroFlow‡ is added in the list of trusted applications.
- Activation of Windows update
- Disable unused interfaces
- Use of this equipment adjacent to or stacked with other equipment should be avoided because it could result in improper operation. If such use is necessary, this equipment and the other equipment should be observed to verify that they are operating normally.
- Use of accessories, transducers and cables other than those specified or provided by Coroventis‡ could result in increased electromagnetic emissions or decreased electromagnetic immunity of this equipment and result in improper operation.
- Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to any part of CoroFlow‡, including cables specified by Coroventis‡. Otherwise, degradation of the performance of this equipment could result.
Precautions:
- The PC and CoroHub‡ shall not be placed within the patient environment (1.5 m from patient).
- For operation of other devices used in conjunction with CoroFlow‡ consult the IFU for each of these devices for details on indication, handling and safety information.
- It is recommended to ensure local routines for data backup of stored recordings. CoroFlow‡ does not create backup of stored data.
- Always check minimum performance requirement on PC to ensure compatibility with CoroFlow‡.
- It is recommended to install CoroFlow‡ on a PC with backup battery to avoid interruption in case of power failure.
- Always manually review and confirm valid cursor positions and detected heart beats.
- Ensure that Pa and Pd pressure waveforms are aligned in phase and offset after equalization, or indices can be mis-calculated.
- Confirm that the correct Wi-Box is selected by manually matching the Wi-Box ID number with the Wi-Box in the lab.
- Changing parameter settings outside of default values may affect measurement performance, only for research purposes.
- Only to be used by healthcare professionals.
- Using a network location to store data may cause previously unidentified risks if the network malfunctions.
- No modification or tampering with CoroFlow‡ is permitted.
- CoroFlow‡, including accessories and components, shall not be used if it has been subject to damage.
- The assembly of medical electrical systems and modifications during the actual service life require evaluation to the requirements according to IEC 60601-1 standard series.
- CoroHub‡ does not have any serviceable parts and require no field maintenance. No modification or tampering with CoroHub‡ is permitted.
- CoroHub‡ shall not be immersed in liquid.
- CoroHub‡ shall not be used if it has been subject to damage.
- PPG values may be non-unique and different combinations or focal/diffuse disease may result in the same PPG value.
- Direct connection to a non-secure network, like the internet, may interfere with correct operation and/or result in inappropriate access to patient information. Furthermore, it should be noted that reconfiguring a used network may lead to inability to import patient as well as export examination data, ultimately leading to a risk of loss of patient and examination data. To avoid this problem Coroventis‡ recommends verifying network settings in the system setup after each change. The same caution is relevant regarding connection to DICOM.
- Always confirm valid pressure tracings, marker positions and selected beats.
- Resetting CoroHub‡ will reset PressureWire connections and Zeroing/Equalization parameters.
MAT-2007904 v4.0
Ultiri™ Measurement System

Indications:
The Ultiri™ Measurement System is intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise, and clinical judgment, to determine if therapeutic intervention is indicated.
Contraindications:
There are no known contraindications for the Ultiri™ Measurement System.
Warnings:
- No modification of this equipment is allowed.
- Prior to use, please review the installed software User Manual and the Instructions for Use supplied with the Ultiri™ Measurement System, Wi-Box™ AO Transmitter, and the PressureWire™ X Guidewire for more information on warnings, precautions / cautions, and set-up instructions.
- Appropriate anticoagulant and vasodilator therapy must be used during the procedure as needed.
- The operator should not touch Ultiri™ Measurement System non-CF connectors (or other non-medical equipment) and the patient or patient leads at the same time. Conductive connection may cause leakage currents to induce ventricular fibrillation.
- High frequency surgical equipment must not be used on a patient at the same time as the PressureWire™ X Guidewire and the Ultiri™ Measurement System.
- Do not use this device for any conditions contraindicated for use of compatible guide wires.
- Inside the catheterization laboratory only port-powered USB drives may be connected to the USB port. Connecting externally powered devices to the USB port in the patient vicinity may compromise electrical isolation and cause patient injury.
- External equipment intended for connection to signal output or other connectors shall comply with relevant IEC standard (e.g., IEC 60601 series for medical electrical equipment). In addition, all such combinations of systems shall comply with the standard IEC 60601-1, Medical electrical equipment - Part 1: General requirements for basic safety and essential performance. Any person who connects external equipment to signal output, or other connectors, has formed a system and is therefore responsible for compliance of the system with the requirements of IEC 60601-1. If in doubt contact a qualified technician. Only the PressureWire™ X Guidewire and the Wi-Box™ AO Transmitter are intended to be used with the Ultiri™ Measurement System wireless receivers.
- Connecting to External Equipment - When used in the patient environment, all equipment connected to the Ultiri™ Measurement System must meet the requirements for medical isolation according to the IEC 60601 safety standards. Connection of equipment that does not follow relevant IEC standards (e.g., IEC 60601 series for medical electrical equipment) may lead to patient injury or death.
- The HDMI output is not isolated. External equipment which is connected to this output must provide isolation against leakage current. The user is responsible for compliance with the requirements of standard IEC 60601- 1, Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
- Electrical Shock Hazard - Do not remove Ultiri™ Measurement System covers. To avoid electrical shock, use only the power supply and power cord supplied with the system. Refer to “Setting Up the System” and “Safety Information” for electrical safety information.
- The main power remains switched on when the system is in Standby mode. Avoid direct or indirect (e.g., via the operator) conductive connection between other electrical equipment and the Ultiri™ Measurement System. Conductive connection may cause leakage currents to induce ventricular fibrillation. High frequency surgical equipment must not be used on a patient at the same time as PressureWire™ X Guidewire and the Ultiri™ Measurement System.
- Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 in) to any part of the Ultiri™ Measurement System, including cables specified by the manufacturer. Otherwise, degradation of the performance of this equipment could result.
- Do not use the Ultiri™ Measurement System if it has been dropped, or in any other way exposed to mechanical or electrical damage, or if liquids are suspected to have penetrated the casing or the power supply unit. This can cause the user to be exposed to electric shock or loss of system function. Contact Abbott Technical Service for further instructions.
- Do not sterilize the Ultiri™ Measurement System or any of its parts. Do not use this system or any of its parts if it has been sterilized.
- The Ultiri™ Measurement System contains a lithium battery for the system real-time clock. Danger of explosion. Battery is not intended to be replaced.
- To prevent possible patient or operator injury and damage to the system, make sure the equipment is securely mounted.
- Make sure the unit is dry before connecting it to the power supply.
- The PressureWire™ X Guidewire is sterilized by ethylene oxide and is intended for one-time use only. Nonpyrogenic. Do not use if the package is opened or damaged. Do not reuse or re-sterilize. Any attempt to reuse or re-sterilize the PressureWire™ X Guidewire may compromise the structural integrity of this device. Adverse effects of using a non-sterile or re-sterilized guide wire may include, but are not limited to: local and / or systemic infection, mechanical damage, inaccurate results.
- Use of this equipment adjacent to or stacked with other equipment should be avoided because it could result in improper operation. If such use is necessary, this equipment and the other equipment should be observed to verify that they are operating normally. If abnormal performance is observed, it is necessary to increase distance between equipment.
- To protect the privacy and security of sensitive information, including electronic protected health information (EPHI), and to protect the integrity of the system itself, the system should be located in a physically secure, access-controlled environment. Do not use the Ultiri™ Measurement System if there is reason to believe the system’s security has been compromised or if the system was unaccounted for during a period of time (i.e., misappropriated, modified, or tampered with).
- The system has no patient alarm functions. Do not use for cardiac monitoring.
Precautions:
- Patients with potential microvascular dysfunction and borderline index values should be interpreted with caution, and management strategies should be guided not only by pressure measurement, but also by possible supplementary clinical risk stratification and other tests.
- Before performing a physiological parameter procedure or creating a physiological parameter recording, review the installed software User Manual for additional warnings and cautions.
- To connect to the correct Wi-Box™ AO Transmitter, you must select the room where the system is being used. The first time you connect to a room, you must enter the room’s information into the system. Refer to the installed software User Manual for more information.
- Do not unplug from AC power or turn off main power until the shutdown is complete and the screen turns black. Disconnecting from AC power before the shutdown is complete may damage the system.
- The power switch shuts down the Ultiri™ Measurement System, but power remains in the mains cable and power supply unit. To fully disconnect from mains power, remove the mains power plug from the wall socket.
- Output sensitivity changes should be performed by a qualified technician. Incorrect settings may result in discrepancies between monitor system and Ultiri™ Measurement System values.
- Direct connection to a non-secure network (e.g., the internet) may interfere with correct operation and / or result in inappropriate access to patient information. Furthermore, it should be noted that reconfiguring a used network may lead to inability to import or export patient examination data, ultimately leading to a risk of loss of patient and examination data. To avoid this problem, Abbott Technical Service recommends verifying network settings in the system setup after each change.
- No connections to other systems or components are to be made to the Ultiri™ Measurement System except through the Connector Panel. No connections are to be made through the Connector Panel except as described in this manual. In addition, all such combinations of systems shall comply with the standard IEC 60601-1, Medical electrical equipment - Part 1: General requirements for basic safety and essential performance. Any person who connects external equipment to the Ultiri™ Measurement System has formed a medical system and is therefore responsible for compliance of the system with the requirements of IEC 60601-1. If in doubt contact a qualified technician. Only the PressureWire™ X Guidewire and the Wi-Box™ AO Transmitter are intended to be used with the Ultiri™ Measurement System.
- PressureWire™ X Guidewire readings may be affected by defibrillation. After defibrillation, restart the procedure. Re-zero and re-equalize the PressureWire™ X Guidewire.
- Radio transmitting equipment, cellular phones, and strong emission sources such as high frequency surgical equipment shall not be used in close proximity to the Ultiri™ Measurement System since this could influence the performance of the device.
Note: The device should be used in a hospital environment except for near active high frequency (HF) surgical equipment and the radio frequency (RF) shielded room of a medical equipment system for magnetic resonance imaging, where the intensity of electromagnetic (EM) disturbances is high. - Check that the monitor cables and aortic pressure transducer (AO) adapter delivered with the Ultiri™ Measurement System interface are compatible with the catheterization laboratory system to be used. The AO should be in accordance with ANSI / AAMI BP22-1994. After the laboratory monitor system has been zeroed, use only the Ultiri™ Measurement System to calibrate the AO and PressureWire™ X Guidewire.
- After use, the PressureWire™ X Guidewire may be a potential biohazard. Handle and dispose of in accordance with accepted medical practice and applicable laws and regulations.
- Do not immerse in liquid: Do not use the Ultiri™ Measurement System if it has been immersed in liquid.
- Ensure that all ventilation holes are unblocked to avoid system overheating and false readings.
Potential Adverse Events:
Potential complications which may be encountered during all catheterization procedures include, but are not limited to:
- Angina
- Arrhythmia
- Bleeding
- Coronary vascular injury
- Death
- Drug reactions to vasodilators (e.g., Adenosine or nitroglycerine) used to induce hyperemia during FFR determination such as bronchospasm, dyspnea, bradycardia, coronary artery spasm
- Hypotension
- Infection
- Ischemia
- Potential allergic reactions to drugs administered for the procedure
- Stenosis
MAT-2208955 v2.0
OPTIS™ and OPTIS™ Next Imaging Systems and Software

INDICATIONS
Applies to OPTIS™ Imaging Systems and Software
The OPTIS™ Software and AptiVue™ E Series Software are intended to be used only with compatible OPTIS™ Imaging Systems.
The OPTIS™ Imaging Systems with a compatible Dragonfly™ Imaging Catheter are intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The compatible Dragonfly™ Imaging Catheters are intended for use in vessels 2.0 to 3.5 mm in diameter. The compatible Dragonfly™ Imaging Catheters are not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
The OPTIS™ Imaging Systems are intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise and clinical judgment to determine if therapeutic intervention is indicated.
Applies to OPTIS™ Next Imaging Systems and Software
The Ultreon™ 1.0 Software and Ultreon™ 2.0 Software are intended to be used only with compatible OPTIS™ Next Imaging Systems.
The OPTIS™ Next Imaging System with a compatible Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
The OPTIS™ Next Imaging Systems are intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise, and clinical judgment to determine if therapeutic intervention is indicated.
Applies to both OPTIS™ and OPTIS™ Next Imaging Systems and Software
The Dragonfly™ OPTIS™ or Dragonfly™ OpStar™ Imaging Catheters are intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly™ OPTIS™ or Dragonfly™ OpStar™ Imaging Catheters are not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
The OPTIS™ and OPTIS™ Next Imaging Systems are intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise, and clinical judgment to determine if therapeutic intervention is indicated.
CONTRAINDICATIONS
The OPTIS™ and OPTIS™ Next Integrated Systems and Mobile Systems with the usage of the OPTIS™ Software, AptiVue™ E Series Software, Ultreon™ 1.0 Software, and Ultreon™ 2.0 Software are contraindicated where introduction of any catheter would constitute a threat to patient safety. Contraindications include:
- Bacteremia or sepsis
- Major coagulation system abnormalities
- Patients diagnosed with coronary artery spasm
- Patients disqualified for coronary artery bypass graft (CABG) surgery
- Patients disqualified for percutaneous transluminal coronary angioplasty (PTCA)
- Severe hemodynamic instability or shock
- Total occlusion
- Large thrombus
- Acute renal failure
- Inability to tolerate systemic anticoagulation is a contraindication to use of OCT for coronary imaging.
- The system has no patient alarm functions. Do not use for cardiac monitoring.
COMPLICATIONS
The following complications may occur as a consequence of intravascular imaging and catheterization procedure:
- Abnormal heart rhythm or arrhythmias
- Acute myocardial infarction
- Allergic reaction to the contrast media or drug administered for the procedure
- Arterial dissection, injury, or perforation
- Bleeding
- Catheter access site reactions: inflammation or granuloma
- Coronary artery spasm
- Death
- Embolism
- Hypotension
- Infection
- Myocardial ischemia
- Renal insufficiency or failure from contrast media use
- Repeat revascularization
- Thrombus formation, abrupt closure, or total occlusion
- Tissue necrosis
- Unstable angina
WARNINGS
- Prior to use, please review the Instructions for Use supplied with the OPTIS™ imaging system, Dragonfly™ Imaging Catheter, Wi-Box™ AO Transmitter and the PressureWire™ guidewire for more information.
- The Dragonfly™ Imaging Catheter is sterilized by ethylene oxide and is intended for one time use only. Non-pyrogenic. Do not use if the package is opened or damaged. Do not reuse or re-sterilize. Any attempt to reuse or re-sterilize may compromise the structural integrity of this device. Adverse effects of using a non-sterile or re-sterilized catheter may include, but are not limited to: local and / or systemic infection, mechanical damage, inaccurate results.
- Appropriate anticoagulant and vasodilator therapy must be used during the procedure as needed.
- Ensure that no air is introduced into the system during the Dragonfly™ Imaging Catheters insertion.
- Observe all advancement and movement of the Dragonfly™ Imaging Catheters under fluoroscopy. Always advance and withdraw the catheter slowly. Failure to observe device movement fluoroscopically may result in vessel injury or device damage. To ensure proper placement, do not move the guide wire after a Dragonfly™ Imaging Catheter is in place.
- If resistance is encountered during advancement or withdrawal of the Dragonfly™ Imaging Catheter, stop manipulation and evaluate under fluoroscopy. If the cause of resistance cannot be determined or mitigated, carefully remove the Dragonfly™ Imaging Catheters and guidewire together as a unit from the patient.
- Leave the guide wire engaged with a Dragonfly™ Imaging Catheter at all times during use. Do not withdraw or advance the guide wire prior to withdrawing the Dragonfly™ Imaging Catheters.
- The Dragonfly™ Imaging Catheters should never be forced into lumens that are narrower than the Dragonfly™ Imaging Catheters body or forced through a tight or heavily calcified lesion.
- The Dragonfly™ Imaging Catheters should not be advanced through abnormally tortuous anatomy.
- When advancing or retracting a Dragonfly™ Imaging Catheter with a monorail tip through a stented vessel, the Dragonfly™ Imaging Catheters may engage the stent between the junction of the Dragonfly™ Imaging Catheters and guide wire, resulting in entrapment of catheter / guide wire, catheter tip separation, stent dislocation, and / or vascular injury.
- Refer to the contrast media Instructions for Use for general warnings and precautions relating to use of contrast media.
- Before creating an OCT recording, review “Performing an OCT Procedure” for additional warnings and cautions in the IFU.
PRECAUTIONS
- Safety and effectiveness have been established for the following patient population: adult patients undergoing non-emergent percutaneous coronary interventions in lesions with reference vessel diameters between 2.0 to 3.5 mm, which are not located in the left main coronary artery or in a target vessel which has undergone previous bypass procedures.
- Follow all instructions, warnings, and cautions provided in “Patient Safety” in the IFU.
- All operators must be knowledgeable in performing OCT and physiological procedures prior to using the OPTIS™ and OPTIS™ Next Integrated Systems and Mobile Systems with the usage of the OPTIS™ Software, AptiVue™ E Series Software, Ultreon™ 1.0 Software, and Ultreon™ 2.0 Software.
- When using saline, heparinized saline is recommended.
- Monitor the OCT image for indications of the Dragonfly™ Imaging Catheters optical failure. If optical failure is suspected, remove the Dragonfly™ Imaging Catheter from the patient, press “Unload” on the drive motor and optical controller (DOC), detach the catheter, and replace it with a new one.
- If the pullback triggers before contrast is injected, repeat the pullback.
- For optimal imaging, only use 100% contrast media.
- Use the minimum flush rate and volume required to image the desired anatomy.
- To obtain accurate measurements, be sure the selection for the Flush Medium is the same as the medium in which you are imaging.
- The Dragonfly™ Imaging Catheters must be purged prior to connection to the DOC to prevent damage to the imaging core.
- Do not insert or remove a Dragonfly™ Imaging Catheter while the DOC is scanning. Do not attempt to disconnect the catheter from the DOC while the “lock” LED is blinking as it could damage the catheter or the DOC. Refer to “Removing the Dragonfly™ Imaging Catheter” in the IFU.
- Never attempt to attach or detach a catheter to the DOC while the "lock" LED is lit.
- Take care in handling the Dragonfly™ Imaging Catheters to prevent breaking the fiber-optics within the catheter. Kinking and bending of the catheter can cause damage. While connecting, ensure the proximal catheter segment is straight and aligned with the DOC. Never attempt to connect and operate the catheter while the catheter remains coiled within the hoop.
- Do not kink, sharply bend, pinch, or crush the Dragonfly™ Imaging Catheters at any time.
- The Dragonfly™ Imaging Catheters have no user serviceable parts. Do not attempt to repair or alter any part of the catheter assembly as provided.
- If you want to make measurements on files that will be exported to standard formats, you must make the measurements BEFORE exporting the images. Using non-OCT software to measure standard format images will not produce accurate measurements.
- Do not use images that have been exported to JPEG or Compressed AVI formats for clinical decision making. These formats use compression methods that may degrade the image quality.
- Artifacts may result in misrepresentation of L-mode data, so L-mode is not recommended for quantification of clinical information.
- It is the user’s responsibility to confirm the lumen contours of all the frames within the reference segment, and to make adjustments if necessary. Red frames indicate low confidence in the detected contours.
- Deleted files cannot be restored. After files have been deleted, they can only be imported back to your system from your archived copies.
- Restoring factory default settings resets ALL user-entered configuration values except the date and time. This button should be used only under the direction of qualified service personnel.
MAT-2309288 v1.0
