Guide PCI Planning and Treatment Decisions With Intravascular Imaging
Optical Coherence Tomography (OCT) is an intravascular imaging modality that uses near-infrared light to provide high-definition, cross-sectional and three-dimensional images of the vessel microstructure.
OCT provides automated, accurate measurements to help guide stent selection, placement and deployment.1 OCT reduces ambiguities impacting vessel prep, stent sizing and expansion to deliver optimal results.2
Intravascular imaging with OCT is performed using Ultreon™ Software, Dragonfly™ Imaging Catheters, and the OPTIS™ Imaging Systems.
Ultreon™ Software
Ultreon™ Software is the next-generation imaging and physiology software powered by AI. Intuitive, fast and efficient: Ultreon™ Software gives better insights to optimize patient outcomes through automation and improved workflow.3-8
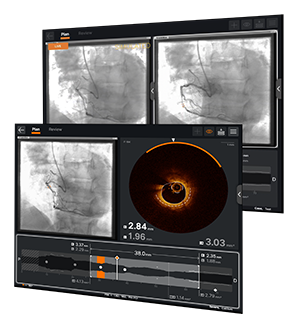
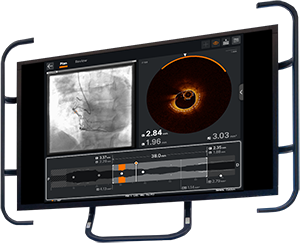
OPTIS™ Next Imaging Systems
The OPTIS™ Next Imaging Systems offer OCT imaging and coronary physiology on one platform with seamless integration into the cath lab and PCI workflow.
Dragonfly OpStar™ Imaging Catheter
Dragonfly OpStar™ Imaging Catheter is the latest generation imaging catheter designed to navigate tortuous anatomy to access distal lesions and provides brighter and higher quality images to achieve true confidence.

References
- Reyes, M. The next innovation in PCI is not a stent. The value of OCT. CathLab Digest. 2019 Oct 6; 27(10).
- Bezerra, H, et al. Analysis of changes in decision-making process during OCT-guided PCI - Insights for the LightLab Initiative. EuroPCR 2020 Presentation.
- Data on file at Abbott.
- Zhang, J, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: The ULTIMATE trial. J Am Coll Cardiol. 2018;72(24):3126-3137.
- Hong, M, et al. IVUS-XPL 5 Year Outcomes, TCT 2019.
- Jones, DA, et al. Angiography alone versus angiography plus optical coherence tomography to guide percutaneous coronary intervention - Outcomes from the Pan-London PCI Cohort. JACC Cardiovasc Interv. 2018 July 23;11(14):1313-1321.
- Cioffi, GM, et al. Does artificial intelligence enhance physician interpretation of optical coherence tomography: Insights from eye tracking. Front. Cardiovasc. Med. 2023 Dec 08;10:1283338.
- Ultreon™ 2.0 Software Instructions for Use (IFU). Refer to IFU for additional information.
MAT-2201010 v2.0
OPTIS™ and OPTIS™ Next Imaging Systems and Software

INDICATIONS
Applies to OPTIS™ Imaging Systems and Software
The OPTIS™ Software and AptiVue™ E Series Software are intended to be used only with compatible OPTIS™ Imaging Systems.
The OPTIS™ Imaging Systems with a compatible Dragonfly™ Imaging Catheter are intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The compatible Dragonfly™ Imaging Catheters are intended for use in vessels 2.0 to 3.5 mm in diameter. The compatible Dragonfly™ Imaging Catheters are not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
The OPTIS™ Imaging Systems are intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise and clinical judgment to determine if therapeutic intervention is indicated.
Applies to OPTIS™ Next Imaging Systems and Software
The Ultreon™ 1.0 Software and Ultreon™ 2.0 Software are intended to be used only with compatible OPTIS™ Next Imaging Systems.
The OPTIS™ Next Imaging System with a compatible Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
The OPTIS™ Next Imaging Systems are intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise, and clinical judgment to determine if therapeutic intervention is indicated.
Applies to both OPTIS™ and OPTIS™ Next Imaging Systems and Software
The Dragonfly™ OPTIS™ or Dragonfly™ OpStar™ Imaging Catheters are intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly™ OPTIS™ or Dragonfly™ OpStar™ Imaging Catheters are not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
The OPTIS™ and OPTIS™ Next Imaging Systems are intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise, and clinical judgment to determine if therapeutic intervention is indicated.
CONTRAINDICATIONS
The OPTIS™ and OPTIS™ Next Integrated Systems and Mobile Systems with the usage of the OPTIS™ Software, AptiVue™ E Series Software, Ultreon™ 1.0 Software, and Ultreon™ 2.0 Software are contraindicated where introduction of any catheter would constitute a threat to patient safety. Contraindications include:
- Bacteremia or sepsis
- Major coagulation system abnormalities
- Patients diagnosed with coronary artery spasm
- Patients disqualified for coronary artery bypass graft (CABG) surgery
- Patients disqualified for percutaneous transluminal coronary angioplasty (PTCA)
- Severe hemodynamic instability or shock
- Total occlusion
- Large thrombus
- Acute renal failure
- Inability to tolerate systemic anticoagulation is a contraindication to use of OCT for coronary imaging.
- The system has no patient alarm functions. Do not use for cardiac monitoring.
COMPLICATIONS
The following complications may occur as a consequence of intravascular imaging and catheterization procedure:
- Abnormal heart rhythm or arrhythmias
- Acute myocardial infarction
- Allergic reaction to the contrast media or drug administered for the procedure
- Arterial dissection, injury, or perforation
- Bleeding
- Catheter access site reactions: inflammation or granuloma
- Coronary artery spasm
- Death
- Embolism
- Hypotension
- Infection
- Myocardial ischemia
- Renal insufficiency or failure from contrast media use
- Repeat revascularization
- Thrombus formation, abrupt closure, or total occlusion
- Tissue necrosis
- Unstable angina
WARNINGS
- Prior to use, please review the Instructions for Use supplied with the OPTIS™ imaging system, Dragonfly™ Imaging Catheter, Wi-Box™ AO Transmitter and the PressureWire™ guidewire for more information.
- The Dragonfly™ Imaging Catheter is sterilized by ethylene oxide and is intended for one time use only. Non-pyrogenic. Do not use if the package is opened or damaged. Do not reuse or re-sterilize. Any attempt to reuse or re-sterilize may compromise the structural integrity of this device. Adverse effects of using a non-sterile or re-sterilized catheter may include, but are not limited to: local and / or systemic infection, mechanical damage, inaccurate results.
- Appropriate anticoagulant and vasodilator therapy must be used during the procedure as needed.
- Ensure that no air is introduced into the system during the Dragonfly™ Imaging Catheters insertion.
- Observe all advancement and movement of the Dragonfly™ Imaging Catheters under fluoroscopy. Always advance and withdraw the catheter slowly. Failure to observe device movement fluoroscopically may result in vessel injury or device damage. To ensure proper placement, do not move the guide wire after a Dragonfly™ Imaging Catheter is in place.
- If resistance is encountered during advancement or withdrawal of the Dragonfly™ Imaging Catheter, stop manipulation and evaluate under fluoroscopy. If the cause of resistance cannot be determined or mitigated, carefully remove the Dragonfly™ Imaging Catheters and guidewire together as a unit from the patient.
- Leave the guide wire engaged with a Dragonfly™ Imaging Catheter at all times during use. Do not withdraw or advance the guide wire prior to withdrawing the Dragonfly™ Imaging Catheters.
- The Dragonfly™ Imaging Catheters should never be forced into lumens that are narrower than the Dragonfly™ Imaging Catheters body or forced through a tight or heavily calcified lesion.
- The Dragonfly™ Imaging Catheters should not be advanced through abnormally tortuous anatomy.
- When advancing or retracting a Dragonfly™ Imaging Catheter with a monorail tip through a stented vessel, the Dragonfly™ Imaging Catheters may engage the stent between the junction of the Dragonfly™ Imaging Catheters and guide wire, resulting in entrapment of catheter / guide wire, catheter tip separation, stent dislocation, and / or vascular injury.
- Refer to the contrast media Instructions for Use for general warnings and precautions relating to use of contrast media.
- Before creating an OCT recording, review “Performing an OCT Procedure” for additional warnings and cautions in the IFU.
PRECAUTIONS
- Safety and effectiveness have been established for the following patient population: adult patients undergoing non-emergent percutaneous coronary interventions in lesions with reference vessel diameters between 2.0 to 3.5 mm, which are not located in the left main coronary artery or in a target vessel which has undergone previous bypass procedures.
- Follow all instructions, warnings, and cautions provided in “Patient Safety” in the IFU.
- All operators must be knowledgeable in performing OCT and physiological procedures prior to using the OPTIS™ and OPTIS™ Next Integrated Systems and Mobile Systems with the usage of the OPTIS™ Software, AptiVue™ E Series Software, Ultreon™ 1.0 Software, and Ultreon™ 2.0 Software.
- When using saline, heparinized saline is recommended.
- Monitor the OCT image for indications of the Dragonfly™ Imaging Catheters optical failure. If optical failure is suspected, remove the Dragonfly™ Imaging Catheter from the patient, press “Unload” on the drive motor and optical controller (DOC), detach the catheter, and replace it with a new one.
- If the pullback triggers before contrast is injected, repeat the pullback.
- For optimal imaging, only use 100% contrast media.
- Use the minimum flush rate and volume required to image the desired anatomy.
- To obtain accurate measurements, be sure the selection for the Flush Medium is the same as the medium in which you are imaging.
- The Dragonfly™ Imaging Catheters must be purged prior to connection to the DOC to prevent damage to the imaging core.
- Do not insert or remove a Dragonfly™ Imaging Catheter while the DOC is scanning. Do not attempt to disconnect the catheter from the DOC while the “lock” LED is blinking as it could damage the catheter or the DOC. Refer to “Removing the Dragonfly™ Imaging Catheter” in the IFU.
- Never attempt to attach or detach a catheter to the DOC while the "lock" LED is lit.
- Take care in handling the Dragonfly™ Imaging Catheters to prevent breaking the fiber-optics within the catheter. Kinking and bending of the catheter can cause damage. While connecting, ensure the proximal catheter segment is straight and aligned with the DOC. Never attempt to connect and operate the catheter while the catheter remains coiled within the hoop.
- Do not kink, sharply bend, pinch, or crush the Dragonfly™ Imaging Catheters at any time.
- The Dragonfly™ Imaging Catheters have no user serviceable parts. Do not attempt to repair or alter any part of the catheter assembly as provided.
- If you want to make measurements on files that will be exported to standard formats, you must make the measurements BEFORE exporting the images. Using non-OCT software to measure standard format images will not produce accurate measurements.
- Do not use images that have been exported to JPEG or Compressed AVI formats for clinical decision making. These formats use compression methods that may degrade the image quality.
- Artifacts may result in misrepresentation of L-mode data, so L-mode is not recommended for quantification of clinical information.
- It is the user’s responsibility to confirm the lumen contours of all the frames within the reference segment, and to make adjustments if necessary. Red frames indicate low confidence in the detected contours.
- Deleted files cannot be restored. After files have been deleted, they can only be imported back to your system from your archived copies.
- Restoring factory default settings resets ALL user-entered configuration values except the date and time. This button should be used only under the direction of qualified service personnel.
MAT-2309288 v1.0
Dragonfly OpStar™ Imaging Catheter

Indications: The Dragonfly OpStar™ Imaging Catheter with the OCT Imaging System is intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The Dragonfly OpStar™ Imaging Catheter is intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly OpStar™ Imaging Catheter is not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
Contraindications: Use of the Dragonfly OpStar™ Imaging Catheter is contraindicated where introduction of any catheter would constitute a threat to patient safety. Contraindications include:
- Acute renal failure
- Bacteremia or sepsis
- Large thrombus
- Major coagulation system abnormalities
- Patients diagnosed with coronary artery spasm
- Patients disqualified for coronary artery bypass graft (CABG) surgery
- Patients disqualified for percutaneous transluminal coronary angioplasty (PTCA)
- Severe hemodynamic instability or shock
- Total occlusion
- Inability to tolerate systemic anticoagulation is a contraindication to use of OCT for coronary imaging
Warnings:
- Appropriate anticoagulant and vasodilator therapy is recommended to be used during the procedure as ordered by the physician.
- The Dragonfly OpStar™ Imaging Catheter is sterilized by ethylene oxide and is intended for one time use only. Nonpyrogenic. Do not use if the package is opened or damaged. Do not reuse or re-sterilize. Any attempt to reuse or re-sterilize may compromise the structural integrity of this device. Adverse effects of using a non-sterile or re-sterilized imaging catheter may include, but are not limited to:
- Local and/or systemic infection
- Mechanical damage
- Inaccurate results
- Note the product "Use by" date on the package.
- Observe all advancement and movement of the Dragonfly OpStar™ Imaging Catheter under fluoroscopy. Always advance and withdraw the catheter slowly and ensure that the guide wire is coaxial to the monorail. Failure to observe device movement fluoroscopically may result in vessel injury or device damage. To ensure proper placement, do not move the guide wire after the Dragonfly OpStar™ Imaging Catheter is in place.
- If resistance is encountered during withdrawal of the Dragonfly OpStar™ Imaging Catheter:
- Stop manipulation and evaluate under fluoroscopy.
- If guide wire prolapse is observed, readvance the catheter, ensure the guide wire is coaxial - to the monorail, and reattempt withdrawal.
- If the cause of resistance cannot be determined or mitigated, carefully remove the Dragonfly OpStar™ Imaging Catheter and guide wire as a unit from the patient and replace the Dragonfly OpStar™ Imaging Catheter and guide wire. Do not reuse the Dragonfly OpStar™ Imaging Catheter and guide wire.
- Leave the guide wire engaged with the Dragonfly OpStar™ Imaging Catheter at all times during use. Do not retract or advance the guide wire prior to withdrawing the Dragonfly OpStar™ Imaging Catheter.
- The Dragonfly OpStar™ Imaging Catheter should never be forced into lumens that are narrower than the catheter body or forced through a tight or heavily calcified lesion.
- The Dragonfly OpStar™ Imaging Catheter should not be advanced through abnormally tortuous anatomy.
- When advancing or retracting a Dragonfly OpStar™ Imaging Catheter with a monorail tip through a stented vessel, the Dragonfly imaging catheter may engage the stent between the junction of the Dragonfly OpStar™ Imaging Catheter and guide wire, resulting in entrapment of the catheter/guide wire, catheter tip separation, stent dislocation and/or vascular injury.
- Do not remove the Dragonfly OpStar™ Imaging Catheter from the DOC until the procedure is complete to avoid a potential sterility breach.
- Always verify that the Dragonfly OpStar™ Imaging Catheter has been properly prepared prior to inserting into vasculature.
- The safety and effectiveness of the coated device has not been established, or is unknown, in vascular regions other than those specifically indicated.
- Failure to abide by the warnings in this Instructions for Use might result in damage to the device coating, which may necessitate intervention or result in serious adverse events.
Precautions:
- Safety and effectiveness have been established for the following patient population: adult patients undergoing non-emergent percutaneous coronary interventions in lesions with reference vessel diameters between 2.0 mm to 3.5 mm, which were not located in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
- Use the minimum flush rate and volume required to image the desired anatomy.
- For optimal imaging, only use 100% contrast media.
- Refer to contrast media Instructions for Use for general warnings and precautions relating to contrast media.
- Do not kink, sharply bend, pinch, or crush the Dragonfly OpStar™ Imaging Catheter at any time.
- The Dragonfly OpStar™ Imaging Catheter has no user serviceable parts. Do not attempt to repair or alter any part of the Dragonfly OpStar™ Imaging Catheter assembly as provided.
- After use, the Dragonfly OpStar™ Imaging Catheter may be a potential biohazard. Handle and dispose of in accordance with accepted medical practice and applicable laws and regulations.
- When using saline, heparinized saline is recommended. When wet, the hydrophilic coating increases the lubricity of the coated surface.
- Avoid abrasion of the hydrophilic coating. Use caution when manipulating, advancing and / or withdrawing these devices through needles, metal cannulas, stents, or other devices with sharp edges, or through tortuous or calcified blood vessels. Manipulation, advancement, and / or withdrawal past sharp or beveled edges may result in destruction and / or separation of the outer coating, which may lead to clinical adverse events, resulting in coating material remaining in the vasculature or device damage. This may result in adverse events requiring additional intervention.
- The integrity and performance of the device coating can be negatively impacted by preparation with incompatible media or solvents. Please take note of the following important recommendations:
- Avoid wiping the device with dry gauze as this may damage the device coating.
- Avoid excessive wiping of the coated device.
- Avoid using alcohol, antiseptic solutions, or other solvents to pre-treat the device because this may cause unpredictable changes in the coating which could negatively affect the safety and performance of the catheter.
- Do not soak the device as it may adversely impact the hydrophilic coating on the catheter.
- The Dragonfly OpStar™ Imaging Catheter must be purged prior to connection to the DOC to prevent damage to the imaging core.
- Ensure that the Dragonfly OpStar™ Imaging Catheter tip marker has been properly identified and differentiated from the lens marker before contrast administration and prior to performing the OCT reading.
- Never attempt to attach or detach the Dragonfly OpStar™ Imaging Catheter to the DOC while the "lock" LED is lit.
Complications:
The following complications may occur as a consequence of intravascular imaging and catheterization procedure:
- Allergic reaction to the contrast media or drug administered for the procedure
- Bleeding
- Arterial dissection, injury or perforation
- Abnormal heart rhythm or arrhythmias
- Unstable angina
- Coronary artery spasm
- Thrombus formation, abrupt closure, or total occlusion
- Embolism
- Infection
- Myocardial ischemia
- Acute myocardial infarction
- Repeat revascularization
- Renal insufficiency or failure from contrast media use
- Death
- Catheter access side reactions: inflammation or granuloma or tissue necrosis
- Hypotension
MAT-2115499 v3.0

