The Assert-IQ™ Insertable Cardiac Monitor (ICM) is now the only ICM system to combine AI, the longest-lasting Bluetooth® ICM* and IQ Insights to optimize patient management across arrhythmia types.4, 13-19
It features:
- Advanced AI algorithms that reduce false positives from AF and Pause episodes while maintaining high sensitivity.4, 5
- Extended monitoring with the longest-lasting Bluetooth® ICM‡ with full functionality and no compromises.
- Clear, crisp EGMs for improved visualization of P-waves.3
- 1.5 Tesla (T) and 3T MR Conditional.
The Assert-IQ ICM system leverages AI to enhance the accuracy of AF and Pause episode detection, significantly reducing data burden for clinicians.4, 5
Longest-Lasting Bluetooth® ICM†
Assert-IQ ICM is a Bluetooth®-enabled ICM that provides reliable, long-lasting cardiac monitoring with no compromises in features.
Assert-IQ™ EL+
6+ years** may be preferred for longer term monitoring, such as those undergoing therapy and at risk of developing further arrhythmias such as AF.
Assert-IQ™ 3+
3+ years** may be preferred for more traditional reasons for monitoring, such as diagnosing syncope, palpitations, or detection of atrial fibrillation (AF) within cryptogenic stroke patients.
The Assert-IQ 3 ICM offers another 3-year battery life option that encapsulates only the essential and most valued ICM features.



Long-term Monitoring for Atrial Fibrillation (AF)
Extend your patient monitoring beyond diagnosis and catheter ablation. Long-term monitoring with an ICM helps detect AF recurrence, even if asymptomatic. 8-12
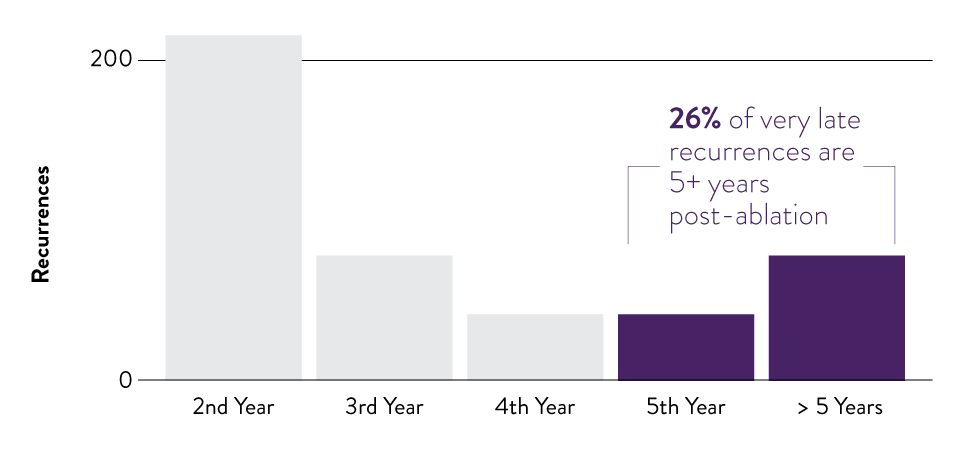
Timing of AF recurrences after the initial catheter ablation for n=2,194 patients that underwent catheter ablation for AF between 2004 and 2019.6
Assert-IQ EL+ is the only ICM that can provide 6+ year monitoring for AF management. 4, 13-19
IQ Insights
Intelligent diagnostics provide trends and timelines of clinically relevant information to provide a holistic view for patient management and empower data-driven decisions.
Premature
Ventricular
Contractions
(PVCs)
Elevated Heart Rate with and without Activity
Body Position &
Posture Changes
DirectTrend™ Viewer



Remote Programmability
Assert-IQ ICM offers simple and secure remote programming, allowing you to control any connected device without requiring your patients to physically visit the clinic.**** This can reduce clinic workflow burden and ensure transmitted data is unique to the patient.
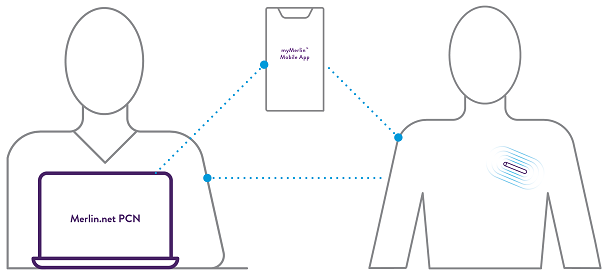
All parameters can be remotely programmed by the clinician.^
Assert-IQ ICM: The Latest Cardiac Monitoring Innovation
Enhance Your ICM Management Skills and Knowledge with Merlin.net™ Patient Care Network (PCN)
Explore a three-part allied health professional series, led by a top expert in Merlin.net PCN and ICM patient management.
Stay Informed
Sign up to hear about our technology, education opportunities, and more.
Read the Latest Blog Article
Stay up to date with recent news, product highlights, and case studies.
References
†As of 12.31.23. Reveal LINQ‡ User Manual, LINQ II‡ User Manual, Lux Dx‡ User Manual, Biomonitor III‡ User Manual, Biomonitor IIIm‡ User Manual, Biomonitor IV‡ User Manual.
*Model DM5500
**Data on File, Abbott - Report 90984075.
***Key Episodes is a feature of Merlin.net™ Patient Care Network.
****Available on select models.
^Not applicable for Name, DOB, and reason for monitoring
- Gopinathannair R, Shehata MM, Afzal MR, et al. Novel Algorithms Improve Arrhythmia Detection Accuracy in Insertable Cardiac Monitors. Journal of Cardiovascular Electrophysiology. 2023;34(9):1961-1968. doi:10.1111/jce.16007
- Gardner RS, Quartieri F, Betts TR, et al. Reducing the Electrogram Review Burden Imposed by Insertable Cardiac Monitors. J Cardiovascular Electrophysiology. 2022;33(4):741-750. doi:10.1111/jce.15397
- Shehata MM, Nair DG, Qu F, et al. Insertable Cardiac Monitor P-wave Visibility in a New Clinical Report. Presented at Asia Pacific Heart Rhythm Society (APHRS); Bangkok Thailand; 2022.
- Abbott. Assert-IQ ICM and Merlin.net Artificial Intelligence for Use with Assert-IQ ICM User Manuals.
- Birgersdotter-Green U, Ojeda W, Manyam H, et al. Atrial Fibrillation Detection Performance of the Assert-IQ Insertable Cardiac Monitor: Results from an Assert-IQ Post-Market Clinical Study and a Novel Artificial Intelligence Algorithm. Heart Rhythm O2. November 11, 2025; DOI: https://doi.org/10.1016/j.hroo.2025.10.021
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. Very Late Recurrence (More Than 1 Year) After AF Ablation. Heart Rhythm. 2017;14(10):e275-e444. doi:10.1016/j.hrthm.2017.05.012.
- Sanchez-Somonte P, Kittichamroen N, Gao-Kang J, et al. Incremental Efficacy for Repeat Ablation Procedures for Catheter Ablation of Atrial Fibrillation: 5-Year Follow-Up. JACC: Advances. 2024;3(9). doi:10.1016/j.jacadv.2024.101200.
- Balabanski T, Brugada J, Arbelo E, et al. Impact of Monitoring on Detection of Arrhythmia Recurrences in the ESC-EHRA EORP Atrial Fibrillation Ablation Long-term Registry. Europace. 2019;21(12):1802-1808. doi:10.1093/europace/euz216.
- Hindricks G, Piorkowski C, Tanner H, et al. Perception of Atrial Fibrillation Before and After Radiofrequency Catheter Ablation: Relevance of Asymptomatic Arrhythmia Recurrence. Circulation. 2005;112(3):307-313. doi:10.1161/CIRCULATIONAHA.104.518837.
- Schirdewan A, Herm J, Roser M, et al. Loop Recorder Detected High Rate of Atrial Fibrillation Recurrence after a Single Balloon- or Basket-Based Ablation of Paroxysmal Atrial Fibrillation: Results of the MACPAF Study. Frontiers in Cardiovascular Medicine. 2017;4:4. doi: 10.3389/fcvm.2017.00004.
- Takigawa M, Takahashi A, Kuwahara T, et al. Long-term Follow-up After Catheter Ablation of Paroxysmal Atrial Fibrillation: The Incidence of Recurrence and Progression of Atrial Fibrillation. Circulation Arrhythmia and Electrophysiology. 2014;7(2):267-273. doi:10.1161/CIRCEP.113.000471.
- Verma A, Champagne J, Sapp J, et al. Discerning the Incidence of Symptomatic and Asymptomatic Episodes of Atrial Fibrillation Before and After Catheter Ablation (DISCERN AF): A Prospective, Multicenter Study. JAMA Internal Medicine. 2013;173(2):149-156. doi:10.1001/jamainternmed.2013.1561.
- Medtronic. REVEAL LINQ‡ LNQ11 Insertable Cardiac Monitor and Patient Assistant PA96000 Clinician Manual. Updated August 26, 2015. Accessed February 23, 2024. https://www.medtronic.com/content/dam/emanuals/crdm/CONTRIB_215651.pdf
- Medtronic. LINQ II‡ LNQ22 Insertable Cardiac Monitor Clinician Manual. Updated September 01, 2022. Accessed February 23, 2024. https://www.medtronic.com/content/dam/emanuals/crdm/M032283C001B_view.pdf
- Boston Scientific. User’s Manual, LUX-Dx‡ Insertable Cardiac Monitor System M301, 2925, 2935. Updated April 2023. Accessed February 23, 2024. https://www.bostonscientific.com/content/dam/elabeling/crm/51656369-001_LUX-Dx_ICM_UM_en_S.pdf
- Boston Scientific. User’s Manual, LUX-Dx II/II+‡ Insertable Cardiac Monitor System M302, M312, 2925, 2929, 2935, 2939. Updated August 2023. Accessed February 23, 2024. https://www.bostonscientific.com/content/dam/elabeling/crm/51583079-001_LUX-Dx_ICM_UM_en_S.pdf
- Biotronik. Technical Manual BIOMONITOR III‡. Updated December 10, 2020. Accessed February 23, 2024. https://manuals.biotronik.com/emanuals-professionals/?country=US&product=ImplCardMon/BioMonitor3/BioMonitor3_US
- Biotronik. Technical Manual BIOMONITOR IIIm‡. Updated December 10, 2020. Accessed February 23, 2024. https://manuals.biotronik.com/emanuals-professionals/?country=US&product=ImplCardMon/BioMonitor3m/BioMonitor3m_US
- Biotronik. Technical Manual BIOMONITOR IV‡. Updated July 17, 2023. Accessed February 23, 2024. https://manuals.biotronik.com/emanuals-professionals/?country=US&product=ImplCardMon/BioMonitor4/BioMonitor4_US
Additional references
- Medtronic. LINQ II‡ LNQ22 Insertable Cardiac Monitor Clinician Manual. Updated September 01, 2022. Accessed February 23, 2024. https://www.medtronic.com/content/dam/emanuals/crdm/M032283C001B_view.pdf
- Boston Scientific. User’s Manual, LUX-Dx‡ Insertable Cardiac Monitor System M301, 2925, 2935. Updated April 2023. Accessed February 23, 2024. https://www.bostonscientific.com/content/dam/elabeling/crm/51656369-001_LUX-Dx_ICM_UM_en_S.pdf
- Boston Scientific. User’s Manual, LUX-Dx II/II+‡ Insertable Cardiac Monitor System M302, M312, 2925, 2929, 2935, 2939. Updated August 2023. Accessed February 23, 2024. https://www.bostonscientific.com/content/dam/elabeling/crm/51583079-001_LUX-Dx_ICM_UM_en_S.pdf
MAT-2303940 v13.0