Every In-Stent Restenosis (ISR) case is unique. Achieving the best outcome for ISR is a multi-step process.

Individualized Solutions for ISR
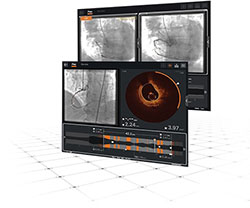
Image - See it Clearly
See ISR clearly with Ultreon™ Software for OCT intravascular imaging.
Intuitive1,2 | Fast2 | Efficient1,2
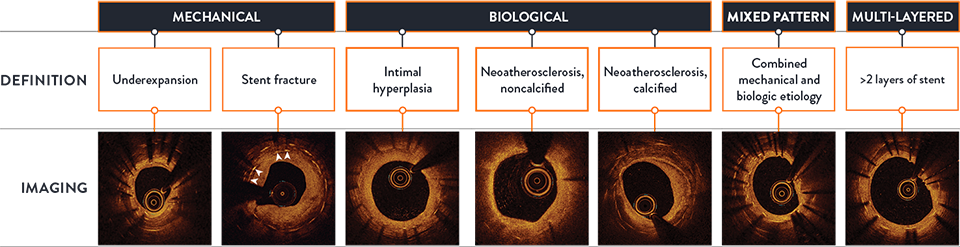
Different types of ISR need different types of treatment, so you need to be sure you can see the true reason for stent failure.3-5
Score - Prepare and Treat the Vessel
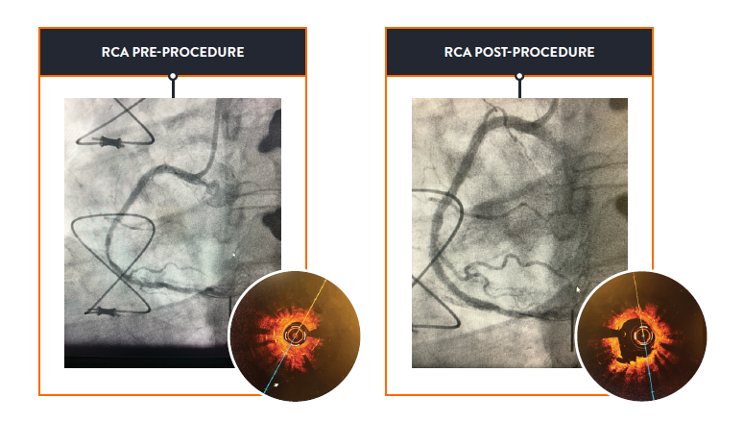
To achieve the largest possible acute lumen gain in your ISR cases, preparing the lesion for treatment is a critical step.
Scoreflex‡ NC Scoring PTCA Catheter offers a vessel preparation and treatment option that may be used for majority of ISR lesions.3,6




References:
- Cioffi GM, et al. Front Cardiovasc Med. 2023;10:1283338.9.
- Ultreon™ 2.0 Software Instructions for Use (IFU). Refer to IFU for additional information.
- Shlofmitz E, et al. Circ Cardiovasc Interv. 2019;12:e007023.
- Shlofmitz E, et al. Cardiovasc Revasc Med. 2021;33:62-67.
- Data on file at Abbott.
- Klein LW, et al. SCAI Expert Consensus Statement on Management of In-Stent Restenosis and Stent Thrombosis. J Soc Cardiovasc Angiogr Interv. 2023;2(4):100971.
- Scoreflex‡ NC Scoring PTCA Catheter Instructions for Use (IFU), Wolverine‡ Cutting Balloon Instructions for Use (IFU), and Philips AngioSculpt‡ EVO RX PTCA Scoring Balloon Catheter Product Brochure. Refer to IFU for additional information.
Scoreflex‡ and OrbusNeich are registered trademarks of OrbusNeich Medical Group Holdings Limited or its affiliates.
Scoreflex‡ NC Scoring PTCA Catheter is manufactured by OrbusNeich Medical Group Holdings Limited or its affiliates. Distributed by CSI Cardiovascular Systems, Inc. CSI is a subsidiary of the Abbott Group of Companies.
CAUTION: This OrbusNeich product is intended for use by or under the direction of a physician. Prior to use, reference the Instructions for Use at eifu.orbusneich.com for more detailed information on Indications, Contraindications, Warnings, Precautions, and Adverse Events.
MAT-2414255 v1.0
Important Safety Information
OPTIS™ Next
Imaging Systems and Software

Indications
The Ultreon™ 2.0 Software is intended to be used only with compatible OPTIS™ Next Imaging Systems.
The OPTIS™ Next Imaging Systems with a compatible Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
The OPTIS™ Next Imaging Systems are intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise, and clinical judgment to determine if therapeutic intervention is indicated.
Contraindications
Use of the Ultreon™ 2.0 Software is contraindicated where introduction of any catheter would constitute a threat to patient safety. Contraindications include:
- Bacteremia or sepsis
- Major coagulation system abnormalities
- Patients diagnosed with coronary artery spasm
- Patients disqualified for coronary artery bypass graft (CABG) surgery
- Patients disqualified for percutaneous transluminal coronary angioplasty (PTCA)
- Severe hemodynamic instability or shock
- Total occlusion
- Large thrombus
- Acute renal failure
- Inability to tolerate systemic anticoagulation is a contraindication to use of OCT for coronary imaging.
- The system has no patient alarm functions. Do not use for cardiac monitoring.
Complications
The following complications may occur as a consequence of intravascular imaging and catheterization procedure:
- Abnormal heart rhythm or arrhythmias
- Acute myocardial infarction
- Allergic reaction to the contrast media or drug administered for the procedure
- Arterial dissection, injury, or perforation
- Bleeding
- Catheter access site reactions: inflammation or granuloma
- Coronary artery spasm
- Death
- Embolism
- Hypotension
- Infection
- Myocardial ischemia
- Renal insufficiency or failure from contrast media use
- Repeat revascularization
- Thrombus formation, abrupt closure, or total occlusion
- Tissue necrosis
- Unstable angina
Warnings
- Prior to use, please review the Instructions for Use supplied with the Dragonfly™ Imaging Catheter for more information.
- Appropriate anticoagulant and vasodilator therapy must be used during the procedure as needed.
- Ensure that no air is introduced into the system during the Dragonfly™ Imaging Catheter insertion.
- Observe all advancement and movement of the Dragonfly™ Imaging Catheter under fluoroscopy. Always advance and withdraw the catheter slowly. Failure to observe device movement fluoroscopically may result in vessel injury or device damage. To ensure proper placement, do not move the guide wire after the Dragonfly™ Imaging Catheter is in place.
- If resistance is encountered during advancement or withdrawal of the Dragonfly™ Imaging Catheter, stop manipulation and evaluate under fluoroscopy. If the cause of resistance cannot be determined or mitigated, carefully remove the Dragonfly™ Imaging Catheter and guidewire together as a unit from the patient.
- Leave the guide wire engaged with the Dragonfly™ Imaging Catheter at all times during use. Do not withdraw or advance the guide wire prior to withdrawing the Dragonfly™ Imaging Catheter.
- The Dragonfly™ Imaging Catheter should never be forced into lumens that are narrower than the Dragonfly™ Imaging Catheter body or forced through a tight or heavily calcified lesion.
- The Dragonfly™ Imaging Catheter should not be advanced through abnormally tortuous anatomy.
- When advancing or retracting a Dragonfly™ Imaging Catheter with a monorail tip through a stented vessel, the Dragonfly™ Imaging Catheter may engage the stent between the junction of the Dragonfly™ Imaging Catheter and guide wire, resulting in entrapment of catheter / guide wire, catheter tip separation, stent dislocation, and / or vascular injury.
- Refer to the contrast media Instructions for Use for general warnings and precautions relating to use of contrast media.
- Before creating an OCT recording, review “Performing an OCT Procedure” for additional warnings and cautions in the IFU.
Precautions
- Safety and effectiveness have been established for the following patient population: adult patients undergoing non-emergent percutaneous coronary interventions in lesions with reference vessel diameters between 2.0 to 3.5 mm, which are not located in the left main coronary artery or in a target vessel which has undergone previous bypass procedures.
- Follow all instructions, warnings, and cautions provided in “Patient Safety” in the IFU.
- All operators must be knowledgeable in performing OCT and physiological procedures prior to using the Ultreon™ 2.0 Software, OPTIS™ Next Imaging System, and the Dragonfly™ Imaging Catheter.
- When using saline, heparinized saline is recommended.
- Monitor the OCT image for indications of Dragonfly™ Imaging Catheter optical failure. If optical failure is suspected, remove the Dragonfly™ Imaging Catheter from the patient, press “Unload” on the drive motor and optical controller (DOC), detach the catheter, and replace it with a new one.
- If the pullback triggers before contrast is injected, repeat the pullback.
- For optimal imaging, only use 100% contrast media.
- Use the minimum flush rate and volume required to image the desired anatomy.
- To obtain accurate measurements, be sure the selection for the Flush Medium is the same as the medium in which you are imaging.
- The Dragonfly™ Imaging Catheter must be purged prior to connection to the DOC to prevent damage to the imaging core.
- Do not insert or remove the Dragonfly™ Imaging Catheter while the DOC is scanning. Do not attempt to disconnect the catheter from the DOC while the “lock” LED is blinking as it could damage the catheter or the DOC. Refer to “Removing the Dragonfly™ Imaging Catheter” in the IFU.
- Never attempt to attach or detach a catheter to the DOC while the "lock" LED is lit.
- Take care in handling the Dragonfly™ Imaging Catheter to prevent breaking the fiber-optics within the catheter. Kinking and bending of the catheter can cause damage. While connecting, ensure the proximal catheter segment is straight and aligned with the DOC. Never attempt to connect and operate the catheter while the catheter remains coiled within the hoop.
- Do not kink, sharply bend, pinch, or crush the Dragonfly™ Imaging Catheter at any time.
- The Dragonfly™ Imaging Catheter has no user serviceable parts. Do not attempt to repair or alter any part of the catheter assembly as provided.
- If you want to make measurements on files that will be exported to standard formats, you must make the measurements BEFORE exporting the images. Using non-OCT software to measure standard format images will not produce accurate measurements.
- Do not use images that have been exported to JPEG or Compressed AVI formats for clinical decision making. These formats use compression methods that may degrade the image quality.
- Artifacts may result in misrepresentation of L-mode data, so L-mode is not recommended for quantification of clinical information.
- It is the user’s responsibility to confirm the lumen contours of all the frames within the reference segment, and to make adjustments if necessary. Red frames indicate low confidence in the detected contours.
- Deleted files cannot be restored. After files have been deleted, they can only be imported back to your system from your archived copies.
- Restoring factory default settings resets ALL user-entered configuration values except the date and time. This button should be used only under the direction of qualified service personnel.
MAT-2306660 v1.0
Dragonfly OpStar™ Imaging Catheter

Indications: The Dragonfly OpStar™ Imaging Catheter with the OCT Imaging System is intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The Dragonfly OpStar™ Imaging Catheter is intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly OpStar™ Imaging Catheter is not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
Contraindications: Use of the Dragonfly OpStar™ Imaging Catheter is contraindicated where introduction of any catheter would constitute a threat to patient safety. Contraindications include:
- Acute renal failure
- Bacteremia or sepsis
- Large thrombus
- Major coagulation system abnormalities
- Patients diagnosed with coronary artery spasm
- Patients disqualified for coronary artery bypass graft (CABG) surgery
- Patients disqualified for percutaneous transluminal coronary angioplasty (PTCA)
- Severe hemodynamic instability or shock
- Total occlusion
- Inability to tolerate systemic anticoagulation is a contraindication to use of OCT for coronary imaging
Warnings:
- Appropriate anticoagulant and vasodilator therapy is recommended to be used during the procedure as ordered by the physician.
- The Dragonfly OpStar™ Imaging Catheter is sterilized by ethylene oxide and is intended for one time use only. Nonpyrogenic. Do not use if the package is opened or damaged. Do not reuse or re-sterilize. Any attempt to reuse or re-sterilize may compromise the structural integrity of this device. Adverse effects of using a non-sterile or re-sterilized imaging catheter may include, but are not limited to:
- Local and/or systemic infection
- Mechanical damage
- Inaccurate results
- Note the product "Use by" date on the package.
- Observe all advancement and movement of the Dragonfly OpStar™ Imaging Catheter under fluoroscopy. Always advance and withdraw the catheter slowly and ensure that the guide wire is coaxial to the monorail. Failure to observe device movement fluoroscopically may result in vessel injury or device damage. To ensure proper placement, do not move the guide wire after the Dragonfly OpStar™ Imaging Catheter is in place.
- If resistance is encountered during withdrawal of the Dragonfly OpStar™ Imaging Catheter:
- Stop manipulation and evaluate under fluoroscopy.
- If guide wire prolapse is observed, readvance the catheter, ensure the guide wire is coaxial - to the monorail, and reattempt withdrawal.
- If the cause of resistance cannot be determined or mitigated, carefully remove the Dragonfly OpStar™ Imaging Catheter and guide wire as a unit from the patient and replace the Dragonfly OpStar™ Imaging Catheter and guide wire. Do not reuse the Dragonfly OpStar™ Imaging Catheter and guide wire.
- Leave the guide wire engaged with the Dragonfly OpStar™ Imaging Catheter at all times during use. Do not retract or advance the guide wire prior to withdrawing the Dragonfly OpStar™ Imaging Catheter.
- The Dragonfly OpStar™ Imaging Catheter should never be forced into lumens that are narrower than the catheter body or forced through a tight or heavily calcified lesion.
- The Dragonfly OpStar™ Imaging Catheter should not be advanced through abnormally tortuous anatomy.
- When advancing or retracting a Dragonfly OpStar™ Imaging Catheter with a monorail tip through a stented vessel, the Dragonfly imaging catheter may engage the stent between the junction of the Dragonfly OpStar™ Imaging Catheter and guide wire, resulting in entrapment of the catheter/guide wire, catheter tip separation, stent dislocation and/or vascular injury.
- Do not remove the Dragonfly OpStar™ Imaging Catheter from the DOC until the procedure is complete to avoid a potential sterility breach.
- Always verify that the Dragonfly OpStar™ Imaging Catheter has been properly prepared prior to inserting into vasculature.
- The safety and effectiveness of the coated device has not been established, or is unknown, in vascular regions other than those specifically indicated.
- Failure to abide by the warnings in this Instructions for Use might result in damage to the device coating, which may necessitate intervention or result in serious adverse events.
Precautions:
- Safety and effectiveness have been established for the following patient population: adult patients undergoing non-emergent percutaneous coronary interventions in lesions with reference vessel diameters between 2.0 mm to 3.5 mm, which were not located in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
- Use the minimum flush rate and volume required to image the desired anatomy.
- For optimal imaging, only use 100% contrast media.
- Refer to contrast media Instructions for Use for general warnings and precautions relating to contrast media.
- Do not kink, sharply bend, pinch, or crush the Dragonfly OpStar™ Imaging Catheter at any time.
- The Dragonfly OpStar™ Imaging Catheter has no user serviceable parts. Do not attempt to repair or alter any part of the Dragonfly OpStar™ Imaging Catheter assembly as provided.
- After use, the Dragonfly OpStar™ Imaging Catheter may be a potential biohazard. Handle and dispose of in accordance with accepted medical practice and applicable laws and regulations.
- When using saline, heparinized saline is recommended. When wet, the hydrophilic coating increases the lubricity of the coated surface.
- Avoid abrasion of the hydrophilic coating. Use caution when manipulating, advancing and / or withdrawing these devices through needles, metal cannulas, stents, or other devices with sharp edges, or through tortuous or calcified blood vessels. Manipulation, advancement, and / or withdrawal past sharp or beveled edges may result in destruction and / or separation of the outer coating, which may lead to clinical adverse events, resulting in coating material remaining in the vasculature or device damage. This may result in adverse events requiring additional intervention.
- The integrity and performance of the device coating can be negatively impacted by preparation with incompatible media or solvents. Please take note of the following important recommendations:
- Avoid wiping the device with dry gauze as this may damage the device coating.
- Avoid excessive wiping of the coated device.
- Avoid using alcohol, antiseptic solutions, or other solvents to pre-treat the device because this may cause unpredictable changes in the coating which could negatively affect the safety and performance of the catheter.
- Do not soak the device as it may adversely impact the hydrophilic coating on the catheter.
- The Dragonfly OpStar™ Imaging Catheter must be purged prior to connection to the DOC to prevent damage to the imaging core.
- Ensure that the Dragonfly OpStar™ Imaging Catheter tip marker has been properly identified and differentiated from the lens marker before contrast administration and prior to performing the OCT reading.
- Never attempt to attach or detach the Dragonfly OpStar™ Imaging Catheter to the DOC while the "lock" LED is lit.
Complications:
The following complications may occur as a consequence of intravascular imaging and catheterization procedure:
- Allergic reaction to the contrast media or drug administered for the procedure
- Bleeding
- Arterial dissection, injury or perforation
- Abnormal heart rhythm or arrhythmias
- Unstable angina
- Coronary artery spasm
- Thrombus formation, abrupt closure, or total occlusion
- Embolism
- Infection
- Myocardial ischemia
- Acute myocardial infarction
- Repeat revascularization
- Renal insufficiency or failure from contrast media use
- Death
- Catheter access side reactions: inflammation or granuloma or tissue necrosis
- Hypotension
MAT-2115499 v3.0
Scoreflex‡ NC Scoring PTCA Catheter

INDICATIONS
The Scoreflex‡ NC Scoring PTCA Catheter is indicated for: Balloon dilatation of a de novo stenotic portion of a coronary artery and in-stent restenosis in coronary arteries in patients evidencing coronary ischemia for the purpose of improving myocardial perfusion.
CONTRAINDICATIONS
The use of the Scoreflex‡ NC Scoring PTCA Catheter is contraindicated in the following patient types:
- Patients with an unprotected left main coronary artery.
- Patients with coronary artery spasm in the absence of a significant stenosis.
WARNINGS
When using this type of device, the following warnings should be observed:
- This device is intended for single use only. Do not resterilize and/or reuse, as this can potentially result in compromised device performance and increased risk of cross-contamination.
- This balloon is not intended for the expansion or delivery of a stent.
- PTCA in patients who are not acceptable candidates for coronary artery bypass graft surgery require careful consideration, including possible hemodynamic support during PTCA, as treatment of this patient population carries special risk.
- To reduce the potential for vessel damage, the inflated diameter of the balloon should approximate the diameter of the vessel just proximal and distal to the stenosis.
- When the catheter is exposed to the vascular system, it should be manipulated while under high-quality fluoroscopic observation. Do not advance or retract the catheter unless the balloon is fully deflated under vacuum. If resistance is met during manipulation, determine the cause of the resistance before proceeding. Applying excessive force to the catheter can result in tip or catheter breakage, catheter kink, or balloon separation.
- Do not twist the catheter shaft in excess of 180 degrees when the tip is constrained.
- Balloon pressure should not exceed the rated burst pressure (RBP) indicated on the package. The rated burst pressure is based on the results of in vitro testing. At least 99.9 percent of the balloons, (with a 95 percent confidence) will not burst at or below their rated burst pressure. Use of a pressure monitoring device is recommended to prevent over pressurization.
- To reduce the potential for air embolus into the vessel, use only the recommended balloon inflation medium. Never use air or any gaseous medium to inflate the balloon.
- Do not re-straighten a kinked hypotube; straightening a kinked metal shaft may result in breakage of the shaft.
- PTCA should only be performed at hospitals where emergency coronary artery bypass graft surgery can be quickly performed in the event of a potentially injurious or life-threatening complication.
PRECAUTIONS
- Use the catheter prior to the “Use By” date specified on the package.
- Prior to angioplasty, the catheter should be examined to verify functionality and ensure that its size and shape are suitable for the specific procedure for which it is to be used.
- The catheter system should be used only by physicians trained in percutaneous transluminal coronary angioplasty.
- During the procedure, appropriate anticoagulant and coronary vasodilator therapy must be provided to the patient as needed. After the procedure, anticoagulant therapy should be continued for a period of time as determined by the physician.
- Never advance the Scoreflex‡ NC Scoring PTCA Catheter without the guidewire extending from the tip.
- Do not use oil-based contrast medium, organic solvents, or alcohols; there is a possibility of catheter leak, damage, or lubrication loss.
- The balloon deflation time has been established as 15 seconds based on in vitro bench testing results.
- Do not reinsert the PTCA catheter into the coil dispenser after procedural use.
- Discard all disposable devices used during this procedure per local requirements for medical device waste disposal.
ADVERSE EVENTS
Adverse events that may be associated with the use of this product include, but are not limited to, the following:
- Death
- Acute myocardial infarction
- Total occlusion of the coronary artery
- Coronary vessel dissection, perforation, rupture, or injury
- Acute vessel closure
- Restenosis of the dilated vessel
- Unstable angina
- Stroke, air embolism and embolization of fragmentation of thrombotic or atherosclerotic material
- Arrhythmias, including ventricular fibrillation
- Hypertension
- Hypotension
- Coronary artery spasm
- Hemorrhage or hematoma
- Arteriovenous fistula
- Drug reactions, allergic reaction to contrast medium
- Infection
- Need for blood transfusion
CAUTION: This product is intended for use by or under the direction of a physician. Prior to use, reference the Instructions for Use, inside the product carton (when available), at manuals.eifu.abbott or at eifu.orbusneich.com for more detailed information on Indications, Contraindications, Warnings, Precautions and Adverse Events. This material is intended for use with healthcare professionals only.
Scoreflex‡ NC Scoring PTCA Catheter is manufactured by OrbusNeich Medical Group Holdings Limited or its affiliates and distributed by Cardiovascular Systems, Inc. (CSI). CSI is a subsidiary of the Abbott Group of Companies.
MAT-2303959 v2.0