





Every complex case is unique, and no lesion is the same. That’s why we focus on providing the therapies, tools, and specialized knowledge that enable you to tailor your approach and optimize long-term, durable outcomes.

Individualized Solutions for Calcified Lesions
Calcified lesions present unique challenges that can compromise stent delivery and expansion.
Abbott’s specialized solutions for coronary calcium empower you in every step from ACCESS to CLOSE:
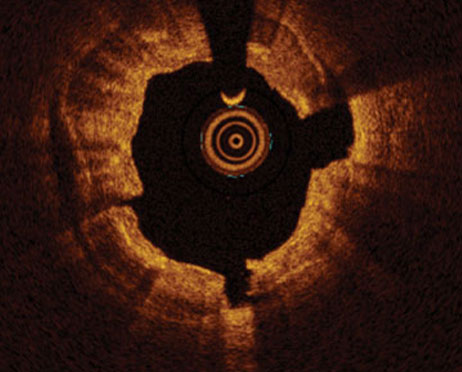
- Assess lesion severity using OCT for clear morphological insights.1
- Prepare and modify tough calcium with dedicated coronary dilatation catheters and atherectomy devices.
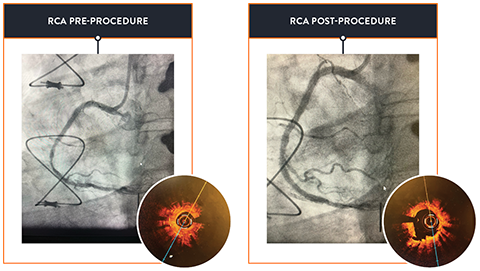
- Achieve durable results2 through thorough lesion preparation and evidence-based therapies, even in complex cases as seen in the results of the pivotal ORBIT II trial.
Individualized Solutions for In-Stent Restenosis
Every In-Stent Restenosis (ISR) case is unique, and achieving the best outcome for ISR is a multi-step process.
Abbott’s individualized solutions from start to finish for ISR will help you achieve optimal outcomes:
- Image: How will you image the vessel?
- Score: How will you prep and treat the vessel?
- Restore: Did you restore the lumen?
Clear
Evaluate lesion morphology using advanced tools that ensure more accurate and informed decisions.
Calculated
Strategize your treatment and remove obstacles with dedicated solutions to achieve optimal clinical preparation.
Confident
Know your choices are backed by data with strong clinical outcomes and real-world evidence.

References:
- Ultreon™ 2.0 Software Instructions for Use (IFU). Refer to IFU for additional information.
- Lee M, et al. Orbital atherectomy for treating de novo, severely calcified coronary lesions: 3-year results of the pivotal ORBIT II trial. Cardiovasc Revasc Med. 2017;18(4):261-264.
MAT-2501743 v1.0
Important Safety Information
OPTIS™ and OPTIS™ Next Imaging Systems and Software

INDICATIONS
Applies to OPTIS™ Imaging Systems and Software
The OPTIS™ Software and AptiVue™ E Series Software are intended to be used only with compatible OPTIS™ Imaging Systems.
The OPTIS™ Imaging Systems with a compatible Dragonfly™ Imaging Catheter are intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The compatible Dragonfly™ Imaging Catheters are intended for use in vessels 2.0 to 3.5 mm in diameter. The compatible Dragonfly™ Imaging Catheters are not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
The OPTIS™ Imaging Systems are intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise and clinical judgment to determine if therapeutic intervention is indicated.
Applies to OPTIS™ Next Imaging Systems and Software
The Ultreon™ 1.0 Software and Ultreon™ 2.0 Software are intended to be used only with compatible OPTIS™ Next Imaging Systems.
The OPTIS™ Next Imaging System with a compatible Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
The OPTIS™ Next Imaging Systems are intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise, and clinical judgment to determine if therapeutic intervention is indicated.
Applies to both OPTIS™ and OPTIS™ Next Imaging Systems and Software
The Dragonfly™ OPTIS™ or Dragonfly™ OpStar™ Imaging Catheters are intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly™ OPTIS™ or Dragonfly™ OpStar™ Imaging Catheters are not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
The OPTIS™ and OPTIS™ Next Imaging Systems are intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise, and clinical judgment to determine if therapeutic intervention is indicated.
CONTRAINDICATIONS
The OPTIS™ and OPTIS™ Next Integrated Systems and Mobile Systems with the usage of the OPTIS™ Software, AptiVue™ E Series Software, Ultreon™ 1.0 Software, and Ultreon™ 2.0 Software are contraindicated where introduction of any catheter would constitute a threat to patient safety. Contraindications include:
- Bacteremia or sepsis
- Major coagulation system abnormalities
- Patients diagnosed with coronary artery spasm
- Patients disqualified for coronary artery bypass graft (CABG) surgery
- Patients disqualified for percutaneous transluminal coronary angioplasty (PTCA)
- Severe hemodynamic instability or shock
- Total occlusion
- Large thrombus
- Acute renal failure
- Inability to tolerate systemic anticoagulation is a contraindication to use of OCT for coronary imaging.
- The system has no patient alarm functions. Do not use for cardiac monitoring.
COMPLICATIONS
The following complications may occur as a consequence of intravascular imaging and catheterization procedure:
- Abnormal heart rhythm or arrhythmias
- Acute myocardial infarction
- Allergic reaction to the contrast media or drug administered for the procedure
- Arterial dissection, injury, or perforation
- Bleeding
- Catheter access site reactions: inflammation or granuloma
- Coronary artery spasm
- Death
- Embolism
- Hypotension
- Infection
- Myocardial ischemia
- Renal insufficiency or failure from contrast media use
- Repeat revascularization
- Thrombus formation, abrupt closure, or total occlusion
- Tissue necrosis
- Unstable angina
WARNINGS
- Prior to use, please review the Instructions for Use supplied with the OPTIS™ imaging system, Dragonfly™ Imaging Catheter, Wi-Box™ AO Transmitter and the PressureWire™ guidewire for more information.
- The Dragonfly™ Imaging Catheter is sterilized by ethylene oxide and is intended for one time use only. Non-pyrogenic. Do not use if the package is opened or damaged. Do not reuse or re-sterilize. Any attempt to reuse or re-sterilize may compromise the structural integrity of this device. Adverse effects of using a non-sterile or re-sterilized catheter may include, but are not limited to: local and / or systemic infection, mechanical damage, inaccurate results.
- Appropriate anticoagulant and vasodilator therapy must be used during the procedure as needed.
- Ensure that no air is introduced into the system during the Dragonfly™ Imaging Catheters insertion.
- Observe all advancement and movement of the Dragonfly™ Imaging Catheters under fluoroscopy. Always advance and withdraw the catheter slowly. Failure to observe device movement fluoroscopically may result in vessel injury or device damage. To ensure proper placement, do not move the guide wire after a Dragonfly™ Imaging Catheter is in place.
- If resistance is encountered during advancement or withdrawal of the Dragonfly™ Imaging Catheter, stop manipulation and evaluate under fluoroscopy. If the cause of resistance cannot be determined or mitigated, carefully remove the Dragonfly™ Imaging Catheters and guidewire together as a unit from the patient.
- Leave the guide wire engaged with a Dragonfly™ Imaging Catheter at all times during use. Do not withdraw or advance the guide wire prior to withdrawing the Dragonfly™ Imaging Catheters.
- The Dragonfly™ Imaging Catheters should never be forced into lumens that are narrower than the Dragonfly™ Imaging Catheters body or forced through a tight or heavily calcified lesion.
- The Dragonfly™ Imaging Catheters should not be advanced through abnormally tortuous anatomy.
- When advancing or retracting a Dragonfly™ Imaging Catheter with a monorail tip through a stented vessel, the Dragonfly™ Imaging Catheters may engage the stent between the junction of the Dragonfly™ Imaging Catheters and guide wire, resulting in entrapment of catheter / guide wire, catheter tip separation, stent dislocation, and / or vascular injury.
- Refer to the contrast media Instructions for Use for general warnings and precautions relating to use of contrast media.
- Before creating an OCT recording, review “Performing an OCT Procedure” for additional warnings and cautions in the IFU.
PRECAUTIONS
- Safety and effectiveness have been established for the following patient population: adult patients undergoing non-emergent percutaneous coronary interventions in lesions with reference vessel diameters between 2.0 to 3.5 mm, which are not located in the left main coronary artery or in a target vessel which has undergone previous bypass procedures.
- Follow all instructions, warnings, and cautions provided in “Patient Safety” in the IFU.
- All operators must be knowledgeable in performing OCT and physiological procedures prior to using the OPTIS™ and OPTIS™ Next Integrated Systems and Mobile Systems with the usage of the OPTIS™ Software, AptiVue™ E Series Software, Ultreon™ 1.0 Software, and Ultreon™ 2.0 Software.
- When using saline, heparinized saline is recommended.
- Monitor the OCT image for indications of the Dragonfly™ Imaging Catheters optical failure. If optical failure is suspected, remove the Dragonfly™ Imaging Catheter from the patient, press “Unload” on the drive motor and optical controller (DOC), detach the catheter, and replace it with a new one.
- If the pullback triggers before contrast is injected, repeat the pullback.
- For optimal imaging, only use 100% contrast media.
- Use the minimum flush rate and volume required to image the desired anatomy.
- To obtain accurate measurements, be sure the selection for the Flush Medium is the same as the medium in which you are imaging.
- The Dragonfly™ Imaging Catheters must be purged prior to connection to the DOC to prevent damage to the imaging core.
- Do not insert or remove a Dragonfly™ Imaging Catheter while the DOC is scanning. Do not attempt to disconnect the catheter from the DOC while the “lock” LED is blinking as it could damage the catheter or the DOC. Refer to “Removing the Dragonfly™ Imaging Catheter” in the IFU.
- Never attempt to attach or detach a catheter to the DOC while the "lock" LED is lit.
- Take care in handling the Dragonfly™ Imaging Catheters to prevent breaking the fiber-optics within the catheter. Kinking and bending of the catheter can cause damage. While connecting, ensure the proximal catheter segment is straight and aligned with the DOC. Never attempt to connect and operate the catheter while the catheter remains coiled within the hoop.
- Do not kink, sharply bend, pinch, or crush the Dragonfly™ Imaging Catheters at any time.
- The Dragonfly™ Imaging Catheters have no user serviceable parts. Do not attempt to repair or alter any part of the catheter assembly as provided.
- If you want to make measurements on files that will be exported to standard formats, you must make the measurements BEFORE exporting the images. Using non-OCT software to measure standard format images will not produce accurate measurements.
- Do not use images that have been exported to JPEG or Compressed AVI formats for clinical decision making. These formats use compression methods that may degrade the image quality.
- Artifacts may result in misrepresentation of L-mode data, so L-mode is not recommended for quantification of clinical information.
- It is the user’s responsibility to confirm the lumen contours of all the frames within the reference segment, and to make adjustments if necessary. Red frames indicate low confidence in the detected contours.
- Deleted files cannot be restored. After files have been deleted, they can only be imported back to your system from your archived copies.
- Restoring factory default settings resets ALL user-entered configuration values except the date and time. This button should be used only under the direction of qualified service personnel.
MAT-2309288 v1.0
Dragonfly OpStar™ Imaging Catheter

Indications: The Dragonfly OpStar™ Imaging Catheter with the OCT Imaging System is intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The Dragonfly OpStar™ Imaging Catheter is intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly OpStar™ Imaging Catheter is not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
Contraindications: Use of the Dragonfly OpStar™ Imaging Catheter is contraindicated where introduction of any catheter would constitute a threat to patient safety. Contraindications include:
- Acute renal failure
- Bacteremia or sepsis
- Large thrombus
- Major coagulation system abnormalities
- Patients diagnosed with coronary artery spasm
- Patients disqualified for coronary artery bypass graft (CABG) surgery
- Patients disqualified for percutaneous transluminal coronary angioplasty (PTCA)
- Severe hemodynamic instability or shock
- Total occlusion
- Inability to tolerate systemic anticoagulation is a contraindication to use of OCT for coronary imaging
Warnings:
- Appropriate anticoagulant and vasodilator therapy is recommended to be used during the procedure as ordered by the physician.
- The Dragonfly OpStar™ Imaging Catheter is sterilized by ethylene oxide and is intended for one time use only. Nonpyrogenic. Do not use if the package is opened or damaged. Do not reuse or re-sterilize. Any attempt to reuse or re-sterilize may compromise the structural integrity of this device. Adverse effects of using a non-sterile or re-sterilized imaging catheter may include, but are not limited to:
- Local and/or systemic infection
- Mechanical damage
- Inaccurate results
- Note the product "Use by" date on the package.
- Observe all advancement and movement of the Dragonfly OpStar™ Imaging Catheter under fluoroscopy. Always advance and withdraw the catheter slowly and ensure that the guide wire is coaxial to the monorail. Failure to observe device movement fluoroscopically may result in vessel injury or device damage. To ensure proper placement, do not move the guide wire after the Dragonfly OpStar™ Imaging Catheter is in place.
- If resistance is encountered during withdrawal of the Dragonfly OpStar™ Imaging Catheter:
- Stop manipulation and evaluate under fluoroscopy.
- If guide wire prolapse is observed, readvance the catheter, ensure the guide wire is coaxial - to the monorail, and reattempt withdrawal.
- If the cause of resistance cannot be determined or mitigated, carefully remove the Dragonfly OpStar™ Imaging Catheter and guide wire as a unit from the patient and replace the Dragonfly OpStar™ Imaging Catheter and guide wire. Do not reuse the Dragonfly OpStar™ Imaging Catheter and guide wire.
- Leave the guide wire engaged with the Dragonfly OpStar™ Imaging Catheter at all times during use. Do not retract or advance the guide wire prior to withdrawing the Dragonfly OpStar™ Imaging Catheter.
- The Dragonfly OpStar™ Imaging Catheter should never be forced into lumens that are narrower than the catheter body or forced through a tight or heavily calcified lesion.
- The Dragonfly OpStar™ Imaging Catheter should not be advanced through abnormally tortuous anatomy.
- When advancing or retracting a Dragonfly OpStar™ Imaging Catheter with a monorail tip through a stented vessel, the Dragonfly imaging catheter may engage the stent between the junction of the Dragonfly OpStar™ Imaging Catheter and guide wire, resulting in entrapment of the catheter/guide wire, catheter tip separation, stent dislocation and/or vascular injury.
- Do not remove the Dragonfly OpStar™ Imaging Catheter from the DOC until the procedure is complete to avoid a potential sterility breach.
- Always verify that the Dragonfly OpStar™ Imaging Catheter has been properly prepared prior to inserting into vasculature.
- The safety and effectiveness of the coated device has not been established, or is unknown, in vascular regions other than those specifically indicated.
- Failure to abide by the warnings in this Instructions for Use might result in damage to the device coating, which may necessitate intervention or result in serious adverse events.
Precautions:
- Safety and effectiveness have been established for the following patient population: adult patients undergoing non-emergent percutaneous coronary interventions in lesions with reference vessel diameters between 2.0 mm to 3.5 mm, which were not located in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
- Use the minimum flush rate and volume required to image the desired anatomy.
- For optimal imaging, only use 100% contrast media.
- Refer to contrast media Instructions for Use for general warnings and precautions relating to contrast media.
- Do not kink, sharply bend, pinch, or crush the Dragonfly OpStar™ Imaging Catheter at any time.
- The Dragonfly OpStar™ Imaging Catheter has no user serviceable parts. Do not attempt to repair or alter any part of the Dragonfly OpStar™ Imaging Catheter assembly as provided.
- After use, the Dragonfly OpStar™ Imaging Catheter may be a potential biohazard. Handle and dispose of in accordance with accepted medical practice and applicable laws and regulations.
- When using saline, heparinized saline is recommended. When wet, the hydrophilic coating increases the lubricity of the coated surface.
- Avoid abrasion of the hydrophilic coating. Use caution when manipulating, advancing and / or withdrawing these devices through needles, metal cannulas, stents, or other devices with sharp edges, or through tortuous or calcified blood vessels. Manipulation, advancement, and / or withdrawal past sharp or beveled edges may result in destruction and / or separation of the outer coating, which may lead to clinical adverse events, resulting in coating material remaining in the vasculature or device damage. This may result in adverse events requiring additional intervention.
- The integrity and performance of the device coating can be negatively impacted by preparation with incompatible media or solvents. Please take note of the following important recommendations:
- Avoid wiping the device with dry gauze as this may damage the device coating.
- Avoid excessive wiping of the coated device.
- Avoid using alcohol, antiseptic solutions, or other solvents to pre-treat the device because this may cause unpredictable changes in the coating which could negatively affect the safety and performance of the catheter.
- Do not soak the device as it may adversely impact the hydrophilic coating on the catheter.
- The Dragonfly OpStar™ Imaging Catheter must be purged prior to connection to the DOC to prevent damage to the imaging core.
- Ensure that the Dragonfly OpStar™ Imaging Catheter tip marker has been properly identified and differentiated from the lens marker before contrast administration and prior to performing the OCT reading.
- Never attempt to attach or detach the Dragonfly OpStar™ Imaging Catheter to the DOC while the "lock" LED is lit.
Complications:
The following complications may occur as a consequence of intravascular imaging and catheterization procedure:
- Allergic reaction to the contrast media or drug administered for the procedure
- Bleeding
- Arterial dissection, injury or perforation
- Abnormal heart rhythm or arrhythmias
- Unstable angina
- Coronary artery spasm
- Thrombus formation, abrupt closure, or total occlusion
- Embolism
- Infection
- Myocardial ischemia
- Acute myocardial infarction
- Repeat revascularization
- Renal insufficiency or failure from contrast media use
- Death
- Catheter access side reactions: inflammation or granuloma or tissue necrosis
- Hypotension
MAT-2115499 v3.0
TREK™ RX & OTW and MINI TREK™ RX & MINI TREK™ II OTW
Coronary Dilatation Catheters

Caution
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE. OBSERVE ALL WARNINGS AND PRECAUTIONS NOTED THROUGHOUT THESE INSTRUCTIONS. FAILURE TO DO SO MAY RESULT IN COMPLICATIONS.
Indications
Applies to TREK™ RX & OTW 2.25 mm – 5.00 mm sizes only:
The TREK™ RX & OTW Coronary Dilatation Catheters are indicated for:
- Balloon dilatation of the stenotic portion of a coronary artery or bypass graft stenosis, for the purpose of improving myocardial perfusion
- Balloon dilatation of a coronary artery occlusion, for the purpose of restoring coronary flow in patients with ST-segment elevation myocardial infarction
- Balloon dilatation of a stent after implantation
Applies to MINI TREK™ RX and MINI TREK™ II OTW
1.50 mm – 2.00 mm sizes only:
The TREK™ RX & OTW Coronary Dilatation Catheters are indicated for:
- Balloon dilatation of the stenotic portion of a coronary artery or bypass graft stenosis, for the purpose of improving myocardial perfusion
- Balloon dilatation of a coronary artery occlusion, for the purpose of restoring coronary flow in patients with ST-segment elevation myocardial infarction
- Balloon dilatation of a stent after implantation (balloon model 2.0 mm only)
- Balloon dilatation of de novo chronic total coronary occlusions (CTO)
Applies to MINI TREK™ RX and MINI TREK™ II OTW 1.20 mm sizes only:
The MINI TREK™ RX and MINI TREK™ II OTW 1.20mm Coronary Dilatation Catheters are indicated for:
- Initial balloon dilatation of the stenotic portion of a coronary artery or bypass graft stenosis (≥ 70% stenosis).
- Balloon dilatation of de novo chronic total coronary occlusions (CTO)
Note (applies to 2.00 mm to 5.00 mm only): Post-deployment stent expansion testing was performed on the bench with the MULTI-LINK VISION™ and MULTI-LINK ULTRA™ stents. All stents should be deployed in accordance with the manufacturer’s indications and instructions for use.
Contraindications (applies to all sizes)
The TREK™ RX & OTW, MINI TREK™ RX and MINI TREK™ II OTW Coronary Dilatation Catheters are not intended to be used to treat patients with:
- An unprotected left main coronary artery
- A coronary artery spasm in the absence of a significant stenosis
Warnings (applies to all sizes)
This device is intended for one time use only. DO NOT resterilize and / or reuse it, as this can compromise device performance and increase the risk of cross contamination due to inappropriate reprocessing.
Percutaneous transluminal coronary angioplasty (PTCA) should only be performed at hospitals where emergency coronary artery bypass graft surgery can bequickly performed in the event of a potentially injurious or life-threatening complication.
PTCA in patients who are not acceptable candidates for coronary artery bypass graft surgery requires careful consideration, including possible hemodynamic support during PTCA, as treatment of this patient population carries special risk.
Use only the recommended balloon inflation medium. Never use air or any gaseous medium to inflate the balloon.
Balloon pressure should not exceed the rated burst pressure (RBP). The RBP is based on results of in vitro testing. At least 99.9% of the balloons (with a 95% confidence) will not burst at or below their RBP. Use of a pressure-monitoring device is recommended to prevent overpressurization.
To reduce the potential for vessel damage, the inflated diameter of the balloon should approximate the diameter of the vessel just proximal and distal to the stenosis.
When the catheter is exposed to the vascular system, it should be manipulated while under high quality fluoroscopic observation. Do not advance or retract the catheter unless the balloon is fully deflated under vacuum. If resistance is met during manipulation, determine the cause of the resistance before proceeding.
Do not use, or attempt to straighten, a catheter if the shaft has become bent or kinked; this may result in the shaft breaking. Instead, prepare a new catheter.
Do not torque the catheter more than one (1) full turn.
Treatment of moderately or heavily calcified lesions is considered to be moderate risk, with an expected success rate of 60 – 85% and increases the risk of acute closure, vessel trauma, balloon burst, balloon entrapment, and associated complications. If resistance is felt, determine the cause before proceeding. Continuing to advance or retract the catheter while under resistance may result in damage to the vessels and / or damage / separation of the catheter.
In the event of catheter damage / separation, recovery of any portion should be performed based on physician determination of individual patient condition and appropriate retrieval protocol.
Precautions (applies to all sizes)
Note the “Use by” date specified on the package.
Inspect all product prior to use. Do not use if the package is open or damaged.
This device should be used only by physicians trained in angiography and PTCA, and / or percutaneous transluminal angioplasty (PTA).
Prior to angioplasty, the dilatation catheter should be examined to verify functionality and ensure that its size is suitable for the specific procedure for which it is to be used.
During the procedure, appropriate anticoagulant and coronary vasodilator therapy must be provided to the patient as needed. Anticoagulant therapy should be continued for a period of time to be determined by the physician after the procedure.
If the surface of the TREK™ RX & OTW, MINI TREK™ RX or MINI TREK™ II OTW Coronary Dilatation Catheter becomes dry, wetting with heparinized normal saline will reactivate the coating.
Do not reinsert the TREK™ RX & OTW, MINI TREK™ RX or MINI TREK™ II OTW Coronary Dilatation Catheter into the coil dispenser after procedural use.
The safety and effectiveness of this PTCA balloon catheter for the treatment of in-stent restenosis (ISR) have not been established.
Applies to TREK™ RX and MINI TREK™ RX only (APPLIES TO ALL SIZES), in addition to above:
The design and construction of these catheters do not provide the user with distal pressure monitoring capability.
Applies to TREK™ RX 4.50mm and5.00mm sizes only, in addition to above:
With 4.5 mm and 5.0 mm balloon dilatation catheters, some increased resistance may be noted upon insertion or withdrawal into or out of the guiding catheter. Choosing a larger guiding catheter size may minimize this.
Applies to TREK™ OTW and MINI TREK™ II OTW (APPLIES TO ALL SIZES), in addition to above:
Bench testing was conducted with 0.014” (.36mm) constant diameter guide wires to establish guide wire compatibility. If another type of guide wire is selected with a different dimensional profile, the compatibility (e.g., wire resistance) should be considered prior to use.
Adverse Events (applies to all sizes)
Possible adverse effects include, but are not limited to, the following:
- Acute myocardial infarction
- Arrhythmias, including ventricular fibrillation
- Arteriovenous fistula
- Coronary artery spasm
- Coronary vessel dissection, perforation, rupture, or injury
- Death
- Drug reactions, allergic reaction to contrast medium
- Embolism
- Hemorrhage or hematoma
- Hypo / hypertension
- Infection
- Restenosis of the dilated vessel
- Total occlusion of the coronary artery or bypass graft
- Unstable angina
MAT-2109405 v2.0
NC TREK NEO™
Coronary Dilatation Catheter

Indications For Use
The NC TREK NEO™ Coronary Dilatation Catheters are indicated for:
a) balloon dilatation of the stenotic portion of a coronary artery or bypass graft stenosis, for the purpose of improving myocardial perfusion
b) balloon dilatation of a coronary artery occlusion, for the purpose of restoring coronary flow in patients with ST-segment elevation myocardial infarction
c) balloon dilatation of a stent after implantation (balloon models 2.00 mm – 5.00 mm only)
Contraindications
The NC TREK NEO™ Coronary Dilatation Catheter is contraindicated for treatment of the unprotected left main coronary artery and for coronary artery spasm in the absence of a significant stenosis
Warnings
This device is intended for one time use only. DO NOT resterilize and / or reuse it, as this can compromise device performance and increase the risk of cross contamination due to inappropriate reprocessing.
Note the “Use by” date specified on the package.
The outside diameter (OD) of the distal 38 cm of the device, including the distal shaft, tip, and the balloon are coated with HYDROCOAT™ Hydrophilic Coating. Refer to PREPARATIONS FOR USE section of these instructions for further information on how to prepare and use this device to ensure it performs as intended. Failure to abide by the warnings in this labeling might result in damage to the device coating, which may necessitate intervention or result in serious adverse events.
Percutaneous transluminal coronary angioplasty (PTCA) should only be performed at centers where emergency coronary artery bypass graft surgery is available.
PTCA in patients who are not acceptable candidates for coronary artery bypass graft surgery requires careful consideration, including possible hemodynamic support during PTCA, as treatment of this patient population carries special risk.
Persons with known history of allergies to any of the components of this device listed below may suffer an allergic reaction to this coronary dilatation catheter. Prior to its use on the patient, the patient should be counseled on the materials contained in the device, and a thorough history of allergies must be discussed. This device contains: polyethylene oxide coating, polyamide, polyether block amide (PEBAX), polyethylene and stainless steel.
Use only the appropriate balloon inflation media. Do not use air or any gaseous medium to inflate the balloon. If gaseous medium is used and balloon rupture occurs there is a potential of causing air embolism and / or vessel injury. Balloon pressure should not exceed the rated burst pressure (RBP). Use of a pressure-monitoring device is recommended to prevent over pressurization.
To reduce the potential for vessel damage, the inflated diameter of the balloon should approximate the diameter of the normal or undiseased vessel segment, just proximal and distal to the stenosis.
Do not use or attempt to straighten a catheter if the shaft has become bent or kinked; this may result in the shaft breaking. Instead, prepare a new catheter.
Treatment of moderately or heavily calcified lesions is considered to be moderate risk, with increase in the risk of acute closure, vessel trauma, balloon burst, balloon entrapment, and associated complications. If resistance is felt, determine the cause before proceeding.
Continuing to advance or retract the catheter while under resistance may result in damage to the vessels and / or damage / separation of the catheter.
Precautions
When the catheter is exposed to the vascular system, it should be manipulated while under high quality fluoroscopic observation. Do not advance or retract the catheter unless the balloon is fully deflated under vacuum. If resistance is met during manipulation, determine the cause of the resistance before proceeding.
Do not torque the catheter more than one (1) full turn.
In the event of catheter damage / separation, retrieval methods (use of additional wires, snares, and / or forceps) may result in additional trauma to the coronary vasculature and / or the vascular access site. Complications may include bleeding, hematoma, or pseudoaneurysm.
To confirm sterility has been maintained, ensure that the package sterile barrier has not been opened or damaged prior to use. Inspect all product and ensure that the device is not damaged. Care must be taken to properly size the balloon prior to use.
During the procedure, appropriate anticoagulant and coronary vasodilator therapy must be provided to the patient as needed. Anticoagulant therapy should be continued for a period of time as determined by the physician after the procedure.
If the surface of the coronary dilatation catheter becomes dry, wet with heparinized normal saline to reactivate the coating.
Do not reinsert the coronary dilatation catheter into the coil dispenser after procedural use
The safety and effectiveness of these devices have not been established, or is unknown, in vascular regions other than those specifically indicated:
- PTCA balloon catheter for the treatment of in-stent restenosis (ISR) has not been established.
- The pediatric population.
- Balloon sizes 1.5 mm, 5.5 mm and 6.0 mm have not been established for balloon dilatation of a stent after implantation.
Potential Adverse Events
Possible adverse effects include, but are not limited to, the following:
- Allergic reaction or hypersensitivity to latex, contrast agent, anesthesia, device materials, and drug reactions to anticoagulation, or antiplatelet drugs
- Vascular access complications which may require transfusion or vessel repair including: Catheter site reactions, Bleeding (ecchymosis, oozing, hematoma, hemorrhage, retroperitoneal hemorrhage), Arteriovenous fistula, pseudoaneurysm, aneurysm, dissection, perforation /rupture, Embolism (air, tissue, plaque, thrombotic material, or device), Peripheral nerve injury, Peripheral ischemia
- Coronary artery or bypass graft complications which may require additional intervention, including:Total occlusion or abrupt closure, Arteriovenous fistula, pseudoaneurysm, aneurysm, dissection, perforation /rupture, Embolism (air, tissue, plaque, thrombotic material, or device), Thrombosis, Stenosis or restenosis
- Pericardial complication which may require additional intervention such as cardiac tamponade, pericardial effusion
- Cardiac arrhythmias (including conduction disorders, atrial and ventricular arrhythmias)
- Cardiac ischemic conditions (including myocardial ischemia, myocardial infarction [including acute], coronary artery spasm and unstable or stable angina pectoris)
- Stroke / cerebrovascular accident (CVA) and transient ischemic attack (TIA)
- System organ failures: Cardio-respiratory arrest, Cardiac failure, Cardiopulmonary failure (including pulmonary edema), Renal insufficiency
- Blood cell disorders (including heparin induced thrombocytopenia)
- Hypertension / hypotension
- Infection
- Nausea and vomiting
- Palpitation, dizziness, and syncope
- Chest pain
- Fever
- Pain
- Death
MAT-2206959 v3.0
Diamondback 360™ and Diamondback 360 Precision™ Coronary Orbital Atherectomy System

Including the Orbital Atherectomy Device (OAD) with GlideAssist™, Saline Pump, ViperWire Advance™ Coronary Guide Wire, and ViperWire Advance™ with Flex Tip Coronary Guide Wire
INDICATIONS
The Diamondback 360™ Coronary Orbital Atherectomy System (OAS) is a percutaneous orbital atherectomy system indicated to facilitate stent delivery in patients with coronary artery disease (CAD) who are acceptable candidates for PTCA or stenting due to de novo, severely calcified coronary artery lesions.
CONTRAINDICATIONS
Use of the OAS is contraindicated for use in the following situations:
- The ViperWire™ guide wire cannot pass across the coronary lesion.
- The target lesion is within a bypass graft or stent.
- The patient is not an appropriate candidate for bypass surgery, angioplasty, or atherectomy therapy.
- The patient has angiographic evidence of thrombus.
- The patient has only one open vessel.
- The patient has angiographic evidence of significant dissection at the treatment site.
- Women who are pregnant or children.
WARNINGS
- Do not use the OAS if the physician does not have experience in coronary angioplasty at their institution.
- Do not use the OAS if the physician does not have training on using the OAS. Contact a CSI representative for information on training.
- Do not use other commercially-available guide wires with the OAD. Only use the Model GWC-12325LG-FLP ViperWire Advance™ Coronary Guide Wire or GWC- 12325LG-FT ViperWire Advance™ Coronary Guide Wire with Flex Tip with the coronary OAD. The ViperWire™ guide wire is designed for use with all coronary OAD crown and shaft configurations.
- Never operate the OAD without normal saline and ViperSlide™ Lubricant. Continually flowing saline and ViperSlide Lubricant is required for cooling and lubricating the OAD during use in order to avoid overheating and permanent damage to the device and possible injury to the patient.
- Do not use the OAD or the ViperWire™ guide wire if their sterile package barriers are compromised or damaged.
- Do not use device during spasm of the vessel.
- Do not re-sterilize or re-use the OAD. If the OAD is re-sterilized or re-used, the OAD may not function properly potentially leading to serious infection and patient harm and/or death.
- Do not re-sterilize or re-use the ViperWire guide wire or the guide wire torquer. If the ViperWire™ guide wire or torquer is re-sterilized or re-used, the guide wire may not function properly potentially leading to serious infection and patient harm and/or death.
- Never use force to advance the spinning crown as vessel perforation may occur. If any resistance to crown travel is felt, reposition the crown away from the lesion, immediately stop treatment, and use fluoroscopy to assess the vessel for any complications. If it is confirmed there are no complications, reposition the device and advance and retract at a targeted rate of 1 to 3 mm per second.
- Use fluoroscopy to monitor and maintain spacing between the driveshaft and guide wire spring tip throughout the procedure. Always keep more than 5mm of spacing between the distal end of the OAD driveshaft and the proximal end of the guide wire spring tip. If the distance between the driveshaft tip and the ViperWire™ guide wire spring tip is insufficient, the driveshaft tip may contact the guide wire spring tip and result in dislodging the guide wire spring tip.
- Immediately stop using any OAS component should mechanical failure of any component occur before or during the atherectomy procedure. Using damaged components may result in OAS malfunction or patient injury.
- Immediately stop use of the OAD if the device stalls. Review for complications if a stall condition occurs. Do not change to high speed if device stalls on low speed.
- Note: If a stall occurs, the On/Off button is inactive for five seconds. If the On/Off button is pressed during this five second lockout period, the lockout period will begin again.
- Initial treatment for each lesion must start at low speed.
- Do not continue treatment if the wire or the device becomes subintimal.
- Do not operate the OAD if there is a bend, kink, or tight loop in the ViperWire™ guide wire. A bend, kink, or tight loop in the ViperWire™ guide wire may cause damage to and malfunctioning of the device during use.
- Performing treatment in excessively tortuous or angulated vessels or bifurcations may result in vessel damage or device failure requiring retrieval.
- Always keep the crown advancing or retracting, while it is orbiting, by continually moving the crown advancer knob to ensure corresponding (1:1) movement between the crown advancer knob and the orbiting crown.
- Do not start or stop orbiting of the crown when tight in a lesion.
- Once the OAD has reached full speed (as indicated by a stable pitch) continue to maintain a targeted travel rate of 1 to 3 mm per second, and do not exceed 10 mm per second. If the orbiting crown remains in one location it may lead to vessel damage.
- Maximum total treatment time should not exceed 5 minutes. If maximum total treatment time exceeds 5 minutes, the OAD shaft, crown, and ViperWire™ guide wire may begin to exhibit signs of wear and result in a device malfunction and possible injury to patient. A team member should track run time during use to verify total run time is not exceeded.
- Do not advance or retract the orbiting crown by advancing the OAD sheath or handle. Buckling of the ViperWire™ guide wire may occur resulting in vessel perforation or vascular trauma. Always advance the orbiting crown by using the crown advancer knob.
- Do not inject contrast solution into the OAD injection port. Device failure or patient harm may occur.
- Do not attempt aspiration through the OAD or saline line while placed within the body. If saline is pulled out through the OAD or saline line, air may enter the system.
- If air is noticed in the system while the OAD is within the body, discontinue treatment by pressing the OAS Pump power button and carefully remove the OAD driveshaft and crown from the introducer sheath/guide catheter.
- Do not allow body parts or clothing to come into contact with spinning components as the OAD orbits at very high speeds. Physical injury to the user or entanglement of clothing with the crown may occur.
- The OAS was only evaluated in severely calcified lesions; therefore the scientific evidence to support use of the OAS to treat other types of lesions/patients is limited.
- Do not spin the crown in GlideAssist™, with the guide wire brake lever in the unlocked position, without first securing the guide wire by holding it with fingers or by using the guide wire torquer. If using the guide wire torquer, ensure that it is securely fastened to the guide wire before starting to spin the crown. Failure to secure the guide wire when the brake is unlocked could allow the guide wire to spin while in GlideAssist™ mode which may result in patient harm.
PRECAUTIONS
- Do not use the OAD if there is damage to the OAD package or if the OAD has reached its shelf-life expiration date.
- If using an adjustable hemostasis valve with the guide catheter, close the hemostasis valve to minimize blood loss from around the guide catheter while still allowing the OAD sheath to slide through the hemostasis valve. Avoid excessive tightening of the hemostasis valve to prevent damaging the OAD catheter sheath. When inserting or removing the OAD crown or drive shaft through the hemostasis valve, use care not to deform the drive shaft.
- If crown and crown advancer knob movements are not moving correspondingly with one another (1:1 movement), retract and re-advance the crown into the lesion using a travel rate between 1 to 3 mm per second. Repeat retracting and advancing the crown into the lesion until crown to crown advancer knob movement correspondence is observed. If the knob and the crown are not moving together, the crown may be driven into the lesion with too much force and may result in the crown springing forward on exiting the lesion.
- Follow standard institution atherectomy policies and procedures, including those related to anticoagulation, channel blockers, and vasodilator therapy.
- Ensure fluoroscopy provides adequate visualization of the OAS system. Always use fluoroscopy to monitor the guide wire spring tip and driveshaft positions at all times throughout the procedure. If wire movement occurs, it is suggested to reposition the guide wire before advancing the device or continuing treatment.
- Applies to Diamondback 360™ Precision Coronary OAS only: Verify that contrast media injections are not above 400 psi and are not occurring during spinning of the crown.
- Applies to Diamondback 360™ Precision Coronary OAS only: If using a guide catheter smaller than 0.071 inches (1.80 mm), contrast media flow may be reduced.
- A temporary pacing lead may be necessary when treating lesions in the right coronary and circumflex arteries due to the possible occurrence of electrophysiological alternations.
- The risk of the occurrence of a dissection or perforation is increased in severely calcified lesions undergoing percutaneous treatment; therefore, on-site surgical back- up should be included as a clinical consideration. If onsite surgical back-up is not provided, then an agreement with an alternative hospital should be considered, in advance of the procedure, where the patient could be transferred in an emergency situation.
- Do not kink or crush the saline tubing as this will reduce the flow of saline and ViperSlide™ Lubricant to the OAD.
- Continually monitor and check the saline tubing and connections for leaks during the procedure.
- Do not spin the crown while advancing or retracting the crown within a guide catheter or tuohy. Damage to the guide catheter, tuohy, and/or OAD may occur.
- Ensure the OAD strain relief remains straight during atherectomy treatment. If the OAD strain relief does not remain straight, the shaft/sheath can kink.
- Do not sterilize the OAS pump. Sterilizing will damage the OAS pump. The OAS pump is intended to be used and maintained outside of the sterile field. Reference the Instructions for Use on cleaning and disinfecting the OAS pump.
- Do not allow fluid to leak onto electrical connections of the OAS pump.
- Do not spin the crown without a seated and supportive guide catheter
- When treating from a larger lumen to a smaller lumen, make sure the guide catheter is coaxial and that the tip of the OAD has entered the coronary artery to control the initial orbit before engaging the crown; engage the OAD tip into the tight stenosis until low speed has reached its treatment potential prior to initiating treatment with high speed.
- To relieve compression in the driveshaft, lock the crown advancer knob at 1cm from the full back position, advance device over wire to a position proximal from the lesion, deploy the guide wire brake, then unlock the crown advancer knob and move it fully proximal. If the OAD is started with existing compression in the driveshaft it may result in the crown springing forward.
- Do not flip contents of tray into sterile field as damage may occur. Components within tray must be carefully removed and placed into sterile field to avoid damage.
- Ejection fractions less than 25% have not been studied, use with low ejection fractions may require additional precautions due to compromised heart function.
POTENTIAL ADVERSE EVENTS
Potential adverse events that may occur and/or require intervention include, but are not limited to:
- Allergic reaction to medication/media/device components. Aneurysm. Angina (ischemic chest pain). Arrhythmias. Arteriovenous fistula. Bleeding. Bruising/hematoma. Cardiac/cardiopulmonary arrest. Cardiac/pericardial tamponade. Cerebrovascular accident (CVA). Death. Embolization, distal (air, tissue, thrombus, device). Emergent coronary artery bypass graft surgery (CABG). Failure to deliver the system to the intended locations. Fever. Heart failure/dysfunction. Hemorrhage, requiring transfusion. Hypotension/hypertension. Infection. Myocardial infarction. Pain. Pericardial effusion. Pseudoaneurysm. Restenosis of treated segment leading to revascularization. Renal insufficiency/failure. Shock (cardiogenic, hypovolemic). Slow flow or no reflow phenomenon. Stroke. Thrombus. Vessel closure, abrupt. Vessel injury, requiring surgical repair. Vessel dissection, perforation, rupture, or spasm. Vessel occlusion.
Diamondback 360™ and Diamondback 360 Precision™ Coronary Orbital Atherectomy System are manufactured and distributed by Cardiovascular Systems, Inc. (CSI). CSI is a subsidiary of the Abbott Group of Companies.
MAT-2303956 v1.0
Scoreflex‡ NC Scoring PTCA Catheter

INDICATIONS
The Scoreflex‡ NC Scoring PTCA Catheter is indicated for: Balloon dilatation of a de novo stenotic portion of a coronary artery and in-stent restenosis in coronary arteries in patients evidencing coronary ischemia for the purpose of improving myocardial perfusion.
CONTRAINDICATIONS
The use of the Scoreflex‡ NC Scoring PTCA Catheter is contraindicated in the following patient types:
- Patients with an unprotected left main coronary artery.
- Patients with coronary artery spasm in the absence of a significant stenosis.
WARNINGS
When using this type of device, the following warnings should be observed:
- This device is intended for single use only. Do not resterilize and/or reuse, as this can potentially result in compromised device performance and increased risk of cross-contamination.
- This balloon is not intended for the expansion or delivery of a stent.
- PTCA in patients who are not acceptable candidates for coronary artery bypass graft surgery require careful consideration, including possible hemodynamic support during PTCA, as treatment of this patient population carries special risk.
- To reduce the potential for vessel damage, the inflated diameter of the balloon should approximate the diameter of the vessel just proximal and distal to the stenosis.
- When the catheter is exposed to the vascular system, it should be manipulated while under high-quality fluoroscopic observation. Do not advance or retract the catheter unless the balloon is fully deflated under vacuum. If resistance is met during manipulation, determine the cause of the resistance before proceeding. Applying excessive force to the catheter can result in tip or catheter breakage, catheter kink, or balloon separation.
- Do not twist the catheter shaft in excess of 180 degrees when the tip is constrained.
- Balloon pressure should not exceed the rated burst pressure (RBP) indicated on the package. The rated burst pressure is based on the results of in vitro testing. At least 99.9 percent of the balloons, (with a 95 percent confidence) will not burst at or below their rated burst pressure. Use of a pressure monitoring device is recommended to prevent over pressurization.
- To reduce the potential for air embolus into the vessel, use only the recommended balloon inflation medium. Never use air or any gaseous medium to inflate the balloon.
- Do not re-straighten a kinked hypotube; straightening a kinked metal shaft may result in breakage of the shaft.
- PTCA should only be performed at hospitals where emergency coronary artery bypass graft surgery can be quickly performed in the event of a potentially injurious or life-threatening complication.
PRECAUTIONS
- Use the catheter prior to the “Use By” date specified on the package.
- Prior to angioplasty, the catheter should be examined to verify functionality and ensure that its size and shape are suitable for the specific procedure for which it is to be used.
- The catheter system should be used only by physicians trained in percutaneous transluminal coronary angioplasty.
- During the procedure, appropriate anticoagulant and coronary vasodilator therapy must be provided to the patient as needed. After the procedure, anticoagulant therapy should be continued for a period of time as determined by the physician.
- Never advance the Scoreflex‡ NC Scoring PTCA Catheter without the guidewire extending from the tip.
- Do not use oil-based contrast medium, organic solvents, or alcohols; there is a possibility of catheter leak, damage, or lubrication loss.
- The balloon deflation time has been established as 15 seconds based on in vitro bench testing results.
- Do not reinsert the PTCA catheter into the coil dispenser after procedural use.
- Discard all disposable devices used during this procedure per local requirements for medical device waste disposal.
ADVERSE EVENTS
Adverse events that may be associated with the use of this product include, but are not limited to, the following:
- Death
- Acute myocardial infarction
- Total occlusion of the coronary artery
- Coronary vessel dissection, perforation, rupture, or injury
- Acute vessel closure
- Restenosis of the dilated vessel
- Unstable angina
- Stroke, air embolism and embolization of fragmentation of thrombotic or atherosclerotic material
- Arrhythmias, including ventricular fibrillation
- Hypertension
- Hypotension
- Coronary artery spasm
- Hemorrhage or hematoma
- Arteriovenous fistula
- Drug reactions, allergic reaction to contrast medium
- Infection
- Need for blood transfusion
CAUTION: This product is intended for use by or under the direction of a physician. Prior to use, reference the Instructions for Use, inside the product carton (when available), at manuals.eifu.abbott or at eifu.orbusneich.com for more detailed information on Indications, Contraindications, Warnings, Precautions and Adverse Events. This material is intended for use with healthcare professionals only.
Scoreflex‡ NC Scoring PTCA Catheter is manufactured by OrbusNeich Medical Group Holdings Limited or its affiliates and distributed by Cardiovascular Systems, Inc. (CSI). CSI is a subsidiary of the Abbott Group of Companies.
MAT-2303959 v2.0
Sapphire‡ NC24 Coronary
Dilatation Catheter

INDICATIONS
The Sapphire‡ NC24 Coronary Dilatation Catheter is indicated for:
- Balloon dilatation of the stenotic portion of a coronary artery or bypass graft stenosis in patients evidencing coronary ischemia for the purpose of improving myocardial perfusion.
- Balloon dilatation of a coronary artery occlusion for the treatment of acute myocardial infarction.
- In-stent restenosis.
- Post-delivery expansion of balloon expandable coronary stents.
CONTRAINDICATIONS
The use of Sapphire‡ NC24 Coronary Dilatation Catheter is contraindicated in the following patient types:
- Patients with an unprotected left main coronary artery.
- Patients with coronary artery spasm in the absence of a significant stenosis.
WARNINGS
When using this type of device, the following warnings should be observed:
- To reduce the potential for vessel damage, the inflated diameter of the balloon should approximate the diameter of the vessel just proximal and distal to the stenosis.
- PTCA in patients who are not acceptable candidates for coronary artery bypass graft surgery require careful consideration, including possible hemodynamic support during PTCA, as treatment of this patient population carries special risk.
- When the catheter is exposed to the vascular system, it should be manipulated while under high quality fluoroscopic observation. Do not advance or retract the catheter unless the balloon is fully deflated under vacuum. If resistance is met during manipulation, determine the cause of the resistance before proceeding.
- Balloon pressure should not exceed the Rated Burst Pressure (RBP) indicated on the package. The RBP is based on the results of in vitro testing. At least 99.9 percent of the balloons, (with a 95 percent confidence) will not burst at or below their rated burst pressure. Use of a pressure monitoring device is recommended to prevent over pressurization.
- PTCA should only be performed at hospitals where emergency coronary artery bypass graft surgery can be quickly performed in the event of a potentially injurious or life-threatening complication.
- Use only the recommended balloon inflation medium. Never use air or any gaseous medium to inflate the balloon.
- This device is designed and intended for single use only. DO NOT reprocess, re-sterilize and/or reuse. Reuse of single-use devices creates a potential risk of patient or user infections. Reuse may lead to impairment of functional performance. Infections and/or limited performance of the device may lead to injury, illness or death in the patient.
- Do not re-straighten a kinked hypotube; straightening a kinked metal shaft may result in breakage of the shaft.
PRECAUTIONS
- Use the catheter prior to the "Use By" date specified on the package.
- Prior to angioplasty, the dilatation catheter should be examined to verify functionality and ensure that its size is suitable for the specific procedure for which it is being used.
- The catheter system should be used only by physicians trained in percutaneous transluminal coronary angioplasty.
- During the procedure, appropriate anticoagulant and coronary vasodilator therapy must be provided to the patient as needed. After the procedure, anticoagulant therapy should be continued for a period of time as determined by the physician.
- Do not re-insert the PTCA catheter into the coil dispenser after procedural use.
- The design and construction of these catheters do not provide the user with distal pressure monitoring capability.
- Discard all disposable devices used during this procedure per local requirements for medical device waste disposal.
- Do not use oil-based contrast medium, organic solvents or alcohols; there is a possibility of catheter leak, damage or lubrication loss.
- Use with caution for procedures involving calcified lesions due to the abrasive nature of these lesions.
POTENTIAL COMPLICATIONS AND ADVERSE EVENTS
Potential complications and adverse effects due to the use of this product include, but are not limited to, the following:
- Death
- Acute myocardial infarction
- Acute Vessel Closure
- Total occlusion of the coronary artery or bypass graft
- Coronary vessel dissection, perforation, rupture, or injury
- Restenosis of the dilated vessel
- Hemorrhage or hematoma
- Unstable angina
- Arrhythmias, including ventricular fibrillation
- Drug reactions, allergic reaction to contrast medium
- Hypo/hypertension
- Infection
- Coronary artery spasm
- Arteriovenous fistula
- Stroke, air embolism and embolization of fragmentation of thrombotic or atherosclerotic material
CAUTION: This product is intended for use by or under the direction of a physician. Prior to use, reference the Instructions for Use, inside the product carton (when available), at manuals.eifu.abbott or at eifu.orbusneich.com for more detailed information on Indications, Contraindications, Warnings, Precautions and Adverse Events. This material is intended for use with healthcare professionals only.
MAT-2400959 v2.0
Sapphire‡ II Pro Balloon
Dilatation Catheter

INDICATIONS
The Sapphire‡ II PRO Dilatation Catheter (Ø1.0-1.25mm configurations) is indicated for:
- balloon pre-dilatation of a stenotic portion of a coronary artery or bypass graft stenosis (≥70% stenosis) for the purpose of improving myocardial perfusion.
The Sapphire‡ II PRO Dilatation Catheter (Ø1.5-4.0mm configurations) is indicated for:
- balloon dilatation of the stenotic portion of a coronary artery or bypass graft stenosis in patients evidencing coronary ischemia for the purpose of improving myocardial perfusion;
- balloon dilatation of a coronary artery occlusion for the treatment of acute myocardial infarction.
The Sapphire‡ II PRO Dilatation Catheter is also indicated for:
- percutaneous transluminal angioplasty in the peripheral vasculature, including renal, femoral, popliteal, infra-popliteal, tibial, and peroneal arteries.
CONTRAINDICATIONS
The use of the Sapphire‡ II PRO Dilatation Catheter is contraindicated:
- for use in patients with an unprotected left main coronary artery.
- for use in patients with coronary artery spasm in the absence of a significant stenosis.
- for use in the neuro vasculature.
- where there is the inability to cross the target lesion with a guidewire.
WARNINGS
When using this type of device, the following warnings should be observed:
- This device is intended for single use only. Do not resterilize and/or reuse, as this can potentially result in compromised device performance and increased risk of cross-contamination.
- The safety and effectiveness of this balloon catheter for the treatment of in stent restenosis (ISR) has not been established.
- This balloon is not intended for the expansion or delivery of a stent.
- To reduce the potential for vessel damage, the inflated diameter of the balloon should approximate the diameter of the vessel just proximal and distal to the stenosis.
- PTCA in patients who are not acceptable candidates for coronary artery bypass graft surgery require careful consideration, including possible hemodynamic support during PTCA, as treatment of this patient population carries special risk.
- When the catheter is exposed to the vascular system, it should be manipulated while under high-quality fluoroscopic observation. Do not advance or retract the catheter unless the balloon is fully deflated under vacuum. If resistance is met during manipulation, determine the cause of the resistance before proceeding. Applying excessive force to the catheter can result in separation of the tip or balloon.
- Balloon pressure should not exceed the rated burst pressure (RBP) indicated on the package. The rated burst pressure is based on the results of in vitro testing. At least 99.9 percent of the balloons, (with a 95 percent confidence) will not burst at or below their rated burst pressure. Use of a pressure monitoring device is recommended to prevent over pressurization.
- To reduce the potential for air embolus into the vessel, use only the recommended balloon inflation medium. Never use air or any gaseous medium to inflate the balloon.
- For the rapid exchange catheter, do no re-straighten a kinked hypotube; straightening a kinked metal shaft may result in breakage of the shaft.
- PTCA should only be performed at hospitals where emergency coronary artery graft surgery can be quickly performed in the event of a potentially injurious or life-threatening complication.
PRECAUTIONS
- Use the catheter prior to the “Use By” date specified on the package.
- Prior to angioplasty, the catheter should be examined to verify functionality and ensure that its size and shape are suitable for the specific procedure for which it is to be used.
- The catheter system should be used only by physicians trained in percutaneous transluminal coronary or peripheral angioplasty.
- During the procedure, appropriate anticoagulant and vasodilator therapy must be provided to the patient as needed. After the procedure, anticoagulant therapy should be continued for a period of time as determined by the physician.
- Do not reinsert the catheter into the coil dispenser after procedural use.
- Discard all disposable devices used during this procedure per local requirements for medical device waste disposal.
- Do not use oil-based contrast medium, organic solvents or alcohols; there is a possibility of catheter leak, damage, or lubrication loss.
- The balloon deflation time has been established as 15 seconds based on in vitro bench testing results.
- Use with caution for procedures involving calcified lesions due to the abrasive nature of these lesions.
ADVERSE EVENTS
Adverse effects due to the use of this product include, but are not limited to, the following:
- Acute myocardial infarction
- Acute or subacute thrombosis
- Acute vessel closure
- Allergic reaction to device, contrast medium, or medication
- Aneurysm
- Arrhythmias, including ventricular fibrillation
- Arteriovenous fistula
- Coronary artery spasm
- Death
- Dissection (perforation, rupture, or injury) of the vessel
- Hemorrhage or hematoma
- Hypertension
- Hypotension
- Infection
- Restenosis of the dilated vessel
- Stroke, air embolism and embolization of fragmentation of thrombotic or atherosclerotic material
- Total occlusion of the artery or bypass graft
- Unstable angina
CAUTION: This product is intended for use by or under the direction of a physician. Prior to use, reference the Instructions for Use, inside the product carton (when available), at manuals.eifu.abbott or at eifu.orbusneich.com for more detailed information on Indications, Contraindications, Warnings, Precautions and Adverse Events. This material is intended for use with healthcare professionals only.
MAT-2400936 v2.0
