包括的機能評価によって、より患者さんに寄り添う診断を
FFRやRFRなどの生理学的検査を実施し、冠動脈狭窄の機能的重症度を評価することができます。1,2
血管造影だけでは評価できない、微小血管抵抗指数(IMR)および冠血流予備能(CFR)の情報を提供し、微小循環機能の診断をサポートします。3,4
包括的な機能評価を通じて、PCIの転帰および患者のQOL改善が期待されています。3, 5-6
PressureWire™ Xガイドワイヤー
PressureWire™ Xガイドワイヤー - 世界で唯一のワイヤレスプレッシャーガイドワイヤーです。1,3,7 冠内圧と温度を測定し、FFR、RFR、IMR、およびCFRを測定できます。

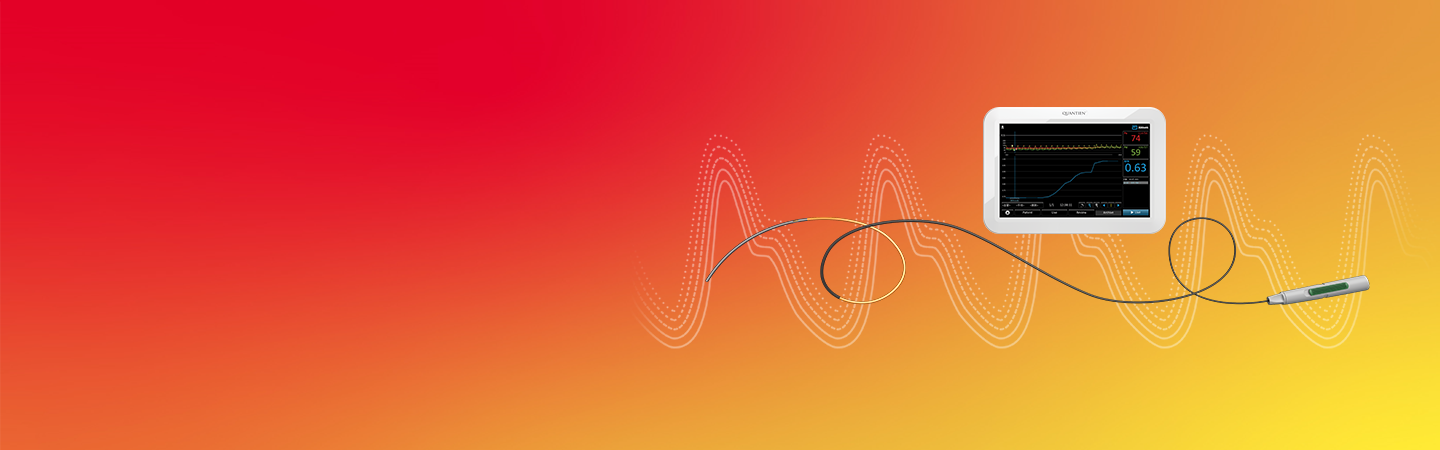
CoroFlow‡ Cardiovascular System
PressureWire™ Xガイドワイヤーとともに使用されるCoroFlow‡ Cardiovascular Systemは、FFR、RFR、IMR、CFRなどの包括的な生理学的指標を測定するためのシステムです。8
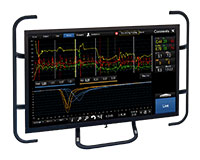
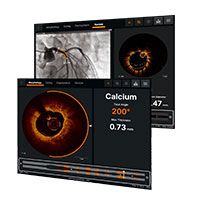
QUANTIEN™ Measurement System
機能的虚血評価のゴールドスタンダードであるFFRに加え、安静時指標のRFRをアボットのワイヤレス冠内圧測定システムで計測可能です。9
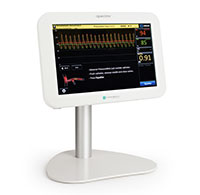
OPTIS™ Imaging Systems
OPTISイメージングシステムは、アンジオ同期機能を備えた血管内光干渉断層法(OCT)画像診断装置です。 OCT画像診断だけでなく、心筋血流予備量比(FFR)や安静時機能的虚血評価(RFR)等の生理学的機能評価も可能なシステムで、ワークフローの効率化に加え、より迅速にPCI手技の最適化を支援します。10
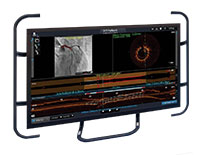
ULTREON™ Software
Ultreon™ ソフトウェアは、カテーテル治療や検査に有用な情報を迅速に提供しPCI の最適化を支援する、次世代のイメージングおよびフィジオロジー ソフトウェアです。合理的で直感的なデザインの Ultreon™ ソフトウェアは、 AI* によるオートメーション機能と改良されたインターフェースで、カテーテル室の標準化および、患者のよりよい転帰に貢献することが期待されます。11-14
*AIは、Ultreon™機能の一部です。
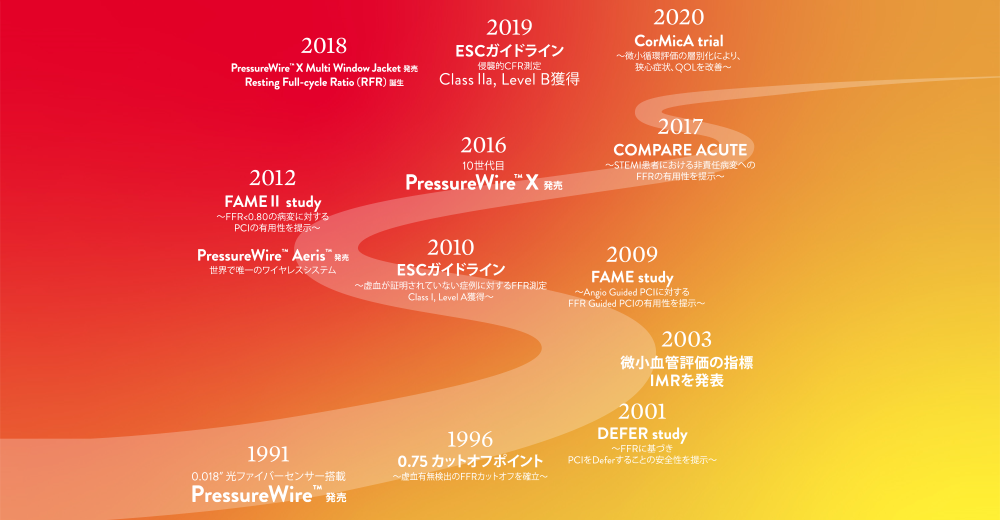
PHYSIOLOGYにおけるアボットの30年にわたる軌跡
アボットは30年にわたり Physiologyに関連する研究を支援し、製品開発と技術を進歩させてきました。
- Physiology その先へ -
アボット はこれからもTotal Physiologyで虚血性心疾患の診断をサポートしていきます。
参照
- PressureWire™ X IFU
- Svanerud J, et al. Validation of a novel non-hyperaemic index of coronary artery stenosis severity: the Resting Full-cycle Ratio (VALIDATE RFR) study. EuroIntervention. 2018;14:806-814.
- Ford T, et al. CorMicA Trial. 2018; 72(23): 2841-55 with online appendix.
- Kunadian V, Chieffo A et al. EHJ & Eurointervention 2020: ehaa503. DOI:10.1093/eurheartj/ehaa503.
- Tonino PA, et al. Angiographics versus functional severity of coronary artery stenoses in the FAME study: Fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol 2010; 55:2816-21.
- De Bruyne B, et al. FFR Guided PCI versus Medical Therapy in Stable Coronary Disease, NEJM. 2012; 367 (11): 991-1001.
- Volcano Corp. Verrata‡ guidewire and PrimeWire Prestige‡ Plus guidewire IFUs, Opsens Inc. OptoWire‡ guidewire and OptoWire‡ II guidewire IFUS, ACIST Medical Systems. Navvus‡ Microcatheter IFU, Boston Scientific Corporation. Comet‡ guidewire IFU.
- Coroventis‡ CoroFlow‡ Cardiovascular System IFU.
- QUANTIEN™ Measurement System IFU.
- OPTIS™ Measurement System IFU
- Data on file at Abbott.
- Zhang J, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: the ULTIMATE trial. J Am Coll Cardiol. 2018;72(24):3126-3137. doi: 10.1016/j.jacc.2018.09.013.
- Hong M, et al. IVUS-XPL 5 Year Outcomes, TCT 2019.
- Jones et al. JACC Cardiovascular Interventions, 2018, vol 11 (14). “Angiography Alone Versus Angiography Plus Optical Coherence Tomography to Guide Percutaneous Coronary Intervention – Outcomes From the Pan-London PCI Cohort”.
MAT-2207794 v3.0