Find a Heart Failure LVAD Clinic
Need help finding an LVAD clinic near you? Our LVAD clinic finder will connect you with an advanced heart failure care team in your area.
The HeartMate 3™ LVAD has been proven to help people with heart failure feel better and live longer.
Meaning that more than half the people who have a HeartMate 3 are still alive at 5 years.

Nearly 60% of the people enrolled in the study were alive after 5 years.
Listen to people tell their HeartMate 3 LVAD stories and how they are living their lives to the fullest.










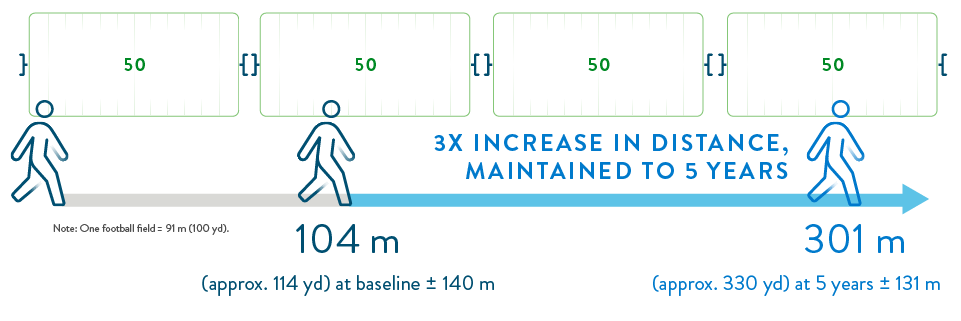
The six-minute walk test is a common way to test for your ability to perform daily physical activities. People who received the HeartMate 3 LVAD had a significant improvement in six-minute walk distance.
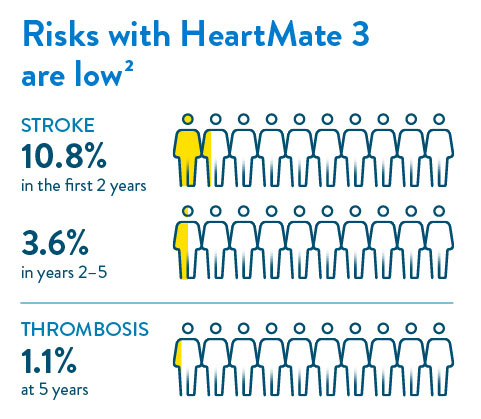
There can be serious complications associated with LVAD therapy. It is important to understand these risks and discuss them with your doctor.
Other than the risks normally associated with major surgery and having general anesthesia, the potential complications of having a HeartMate 3 LVAD are listed here.
Results based on published data from multicenter experience and separate studies, which may involve different patient populations and other variables. Not a head-to-head comparison. Data presented for informational purposes only.
These materials are not intended to replace your doctor’s advice or information. For any questions or concerns you may have regarding the medical procedures, devices and/or your personal health, please discuss these with your physician.



MAT-2406044 v3.0